

Background: Herpes Zoster (HZ) is a painful disease caused by reactivation of Varicella Zoster virus (VZV) [1]. Patients with rheumatoid arthritis (RA), in particular patients treated with Janus Kinase inhibitors (JAKi), are at increased risk of HZ [2, 3]. A subunit vaccine against HZ (HZ/su), consisting of recombinant VZV glycoprotein E (gE) and an adjuvant system, has been shown to induce long-lasting gE-specific antibody responses in healthy adults ≥50 years of age, with levels declining over time but remaining higher than pre-vaccination levels up to at least 5-7 years after vaccination [4]. We previously showed that for patients with RA treated with JAKi, the HZ/su vaccine was safe and induced a serologic response with increased gE-specific antibody levels approximately 6 weeks after vaccination, although with a lower response than healthy controls [5]. So far, there is no data on the long-term immunogenicity to the HZ/su vaccine for patients with RA treated with JAKi.
Objectives: To investigate long-term serologic immunogenicity to the HZ/su vaccine among patients with RA treated with JAKi, as compared to healthy controls.
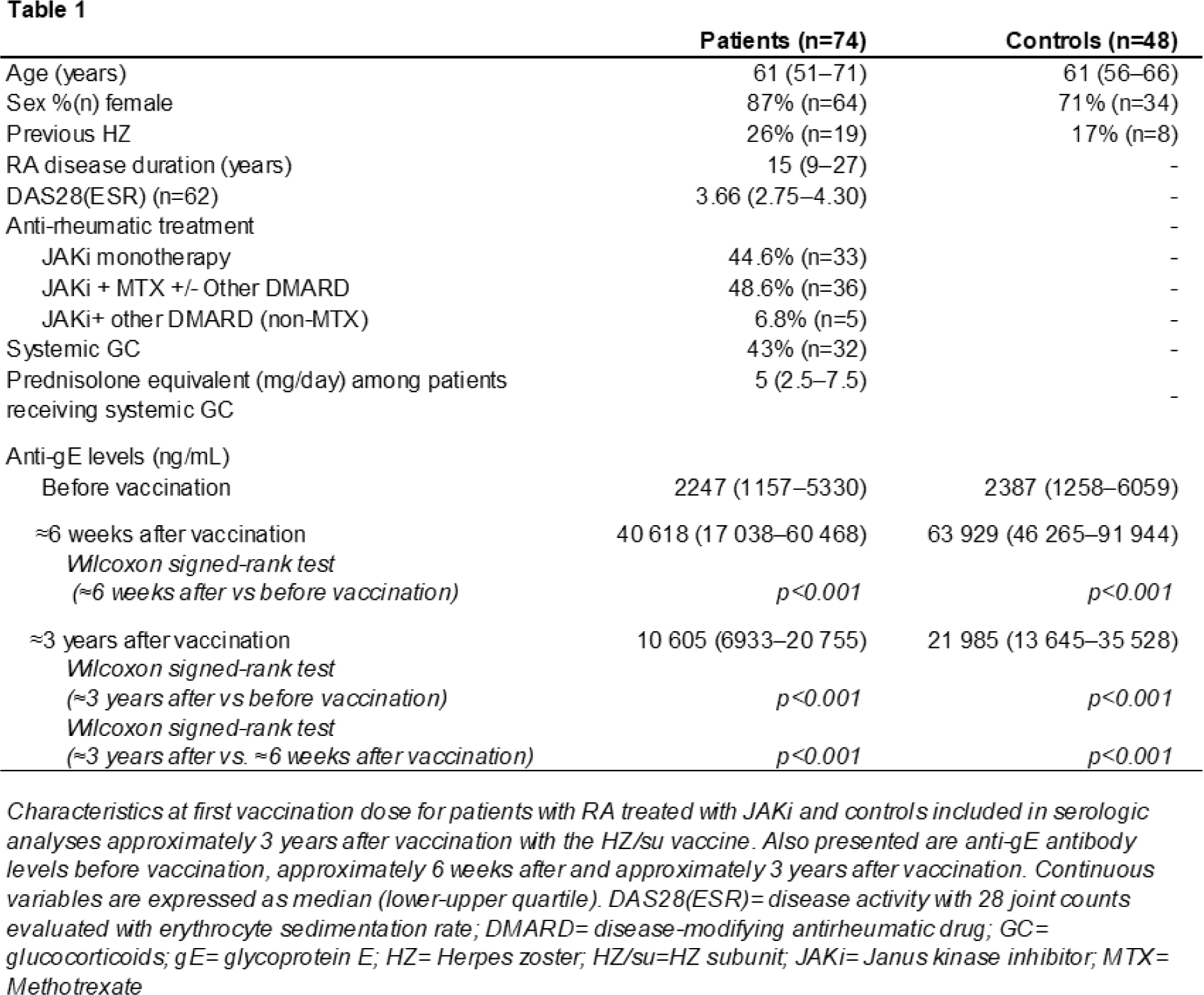
Methods: RA patients treated with JAKi (n=82) at the Department of Rheumatology, Skåne University Hospital, Sweden, and healthy controls (n=51) received two doses of the HZ/su vaccine, as previously described [6]. Serum samples were collected before vaccination, approximately 6 weeks after vaccination and approximately 3 years after vaccination. Anti-gE IgG antibody levels collected 3 years after vaccination were measured using an indirect ELISA and compared to those taken before vaccination and 6-weeks post-vaccination using Wilcoxon Signed-Rank test. Three years post-vaccination levels were compared between patients and controls using an analysis of covariance, adjusted for pre-vaccination levels. Self-reported HZ occurring after the second vaccination dose were analyzed.
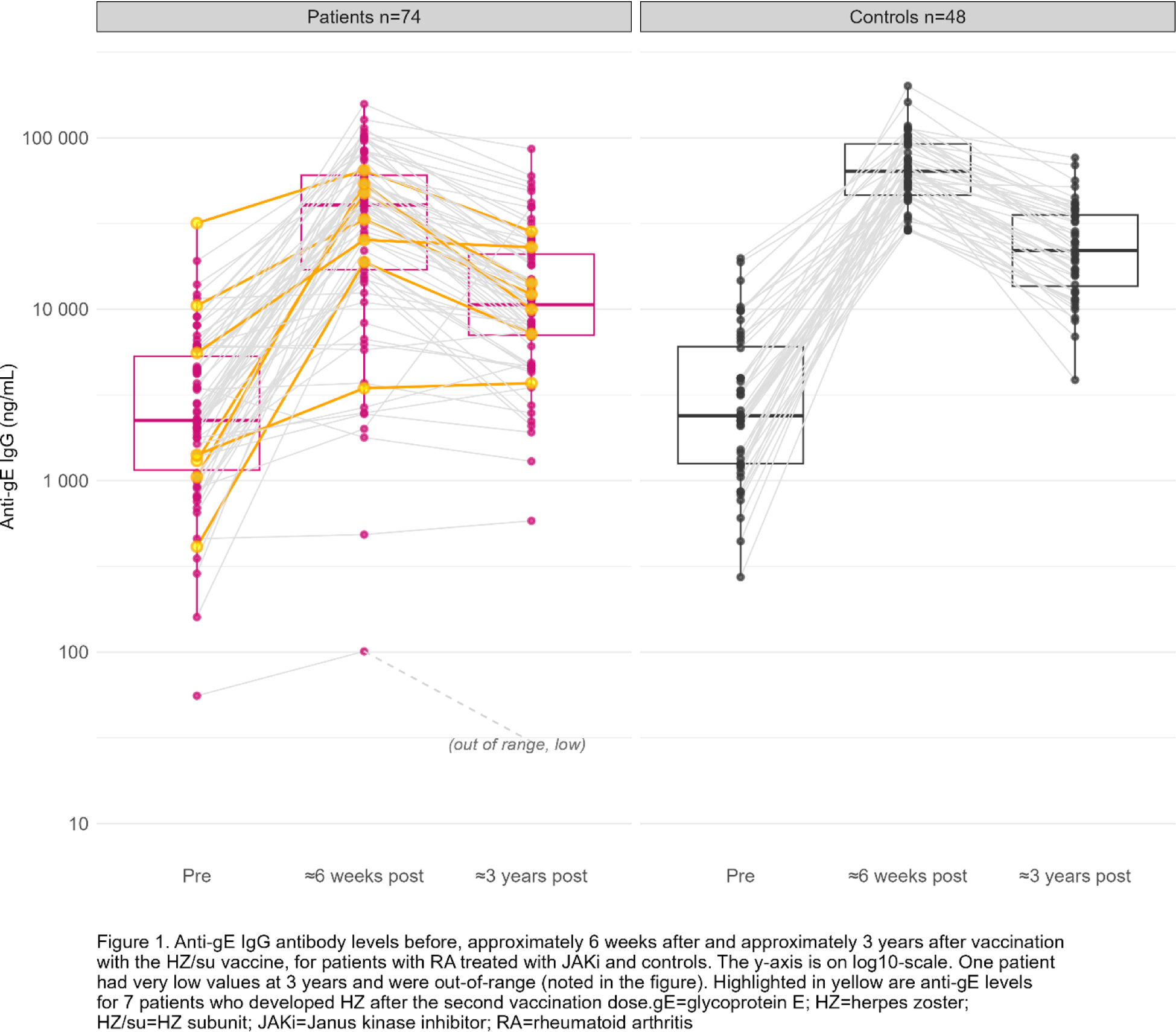
Results: Serum samples from 74 patients and 48 controls collected ≈3 years after vaccination were analysed (Table 1). Three years after vaccination, anti-gE levels were significantly higher than pre-vaccination levels (p<0.001) for both patients and controls but significantly lower than 6-weeks post-vaccination (p<0.001 for both patients and controls) (Table 1; Figure 1). The anti-gE levels at 3 years were higher than pre-vaccination levels for 91% (n=67) of patients and 100% of controls. At 3 years, 47% (n=35) of patients and 77% (n=37) of controls had ≥4-fold higher anti-gE levels as compared to before vaccination. The median fold-change from pre-vaccination levels to 3 years after vaccination was 3.5 (interquartile range 2.1-8.7) for patients, and 7.1 (interquartile range 4.1-15.9) for controls. The geometric mean concentration of anti-gE levels at 3 years was 9521 ng/mL among patients and 21875 ng/mL among controls. The anti-gE-levels 3 years after vaccination were significantly lower among patients than controls (ratio 0.47, 95% CI 0.32-0.68, p<0.001, adjusted for pre-vaccination, levels). Seven patients (9% of 76) and 0 controls reported HZ after the second vaccination dose, resulting in a HZ incidence of 2.82/100 person-years among patients (95% CI 1.13-5.81). Anti-gE levels for the patients who developed HZ are highlighted in Figure 1.
Conclusion: Two doses of the HZ/su vaccine induced long-term increases in anti-gE antibody levels lasting for at least approximately 3 years in most patients with RA treated with JAKi, although with a generally lower response as compared to healthy controls and with anti-gE levels decreasing over time. Despite the vaccination, 9% of patients reported HZ within the first 4 years after vaccination.
REFERENCES: [1] Hope-Simpson RE. THE NATURE OF HERPES ZOSTER: A LONG-TERM STUDY AND A NEW HYPOTHESIS. Proc R Soc Med. 1965;58(1):9-20.
[2] Yun H, Yang S, Chen L, Xie F, Winthrop K, Baddley JW, et al. Risk of Herpes Zoster in Autoimmune and Inflammatory Diseases: Implications for Vaccination. Arthritis Rheumatol. 2016;68(9):2328-37.
[3] Curtis JR, Xie F, Yun H, Bernatsky S, Winthrop KL. Real-world comparative risks of herpes virus infections in tofacitinib and biologic-treated patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75(10):1843-7.
[4] Boutry C, Hastie A, Diez-Domingo J, Tinoco JC, Yu CJ, Andrews C, et al. The Adjuvanted Recombinant Zoster Vaccine Confers Long-Term Protection Against Herpes Zoster: Interim Results of an Extension Study of the Pivotal Phase 3 Clinical Trials ZOE-50 and ZOE-70. Clin Infect Dis. 2022;74(8):1459-67.
[5] Källmark H, Bergström T, Nagel J, Gullstrand B, Einarsson JT, Bengtsson AA, Kapetanovic MC. Serologic immunogenicity and safety of herpes zoster subunit vaccine in patients with rheumatoid arthritis receiving Janus kinase inhibitors. Rheumatology (Oxford). 2024;63(7):2024-33.
Acknowledgements: 1) Meliha C Kapetanovic received support for this study through payments made to the institution by: Unrestricted research grants from Pfizer; Anna-Greta Crafoords Stiftelse för Reumatologisk Forskning; Stiftelsen Konung Gustaf V:s 80-årsfond; Stiftelsen Professor Nanna Svartz Fond; Kockska stiftelser; Södra regionens forskningsstöd; Reumatikerfonden; Greta och Johan Kocks stiftelser; Alfred Österlunds stiftelse; Syskonen Lundgrens stiftelse; SUS donationsfonder; Inger Bendix stiftelse. 2) Tomas Bergström received research funds from Sahlgrenska University Hospital (ALFGBG-717761 and ALFGBG-965849) and has received payment or honoraria for giving lectures on Herpes Zoster for GlaxoSmithKline. 3) Hanna Källmark received research funds by Swedish governmental funding of clinical research (ALF).
Disclosure of Interests: Hanna Källmark: None declared, Tomas Bergström Speaker on Herpes Zoster for GlaxoSmithKline, Diagnostic work for AstraZeneca on COVID-19, Maria Johansson: None declared, Jon Thorkell Einarsson: None declared, Meliha C Kapetanovic Unrestricted research grants from Pfizer.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (