

Background: Autoimmune rheumatological diseases (ARDs) are chronic and complex systemic diseases with a prevalence variation by region, affecting 4% of the population in Spain, 75% of whom are women [1]. Their pathogenic mechanism is not exactly known, but they are considered the result of an imbalance in the regulatory mechanisms of the immune system, with the participation of genetic and environmental factors such as lifestyle, nutrition, drugs and infections [1]. The human body is a symbiotic ecosystem inhabited by trillions of microorganisms living together in niches or microhabitats forming the human microbiota (HM). This microbiota varies in composition and abundance, between and within individuals, and plays a crucial role in various biological functions [2]. At the gut level, homeostasis is maintained through a mutualistic relationship between the gut microbiota (GM) and the host immune system [1]. When this relationship is disrupted, gut dysbiosis can occur, characterised by a reduction in microbial diversity and an overgrowth of opportunistic pathogenic microorganisms. The imbalance in gut homeostasis can trigger an anti-host immune response, which has been associated with the development of autoimmune diseases [4]. The GM is involved in the processing of exogenous substances at the digestive level, such as drugs, taking place an interaction in which the drug alters the composition and function of the GM, and the GM directly or indirectly modifies the chemical structure of the drug and thus its pharmacokinetics and pharmacodynamics [5]. Pharmacomicrobiomics is the field that studies how variations in the GM influence drug action. Studies have shown that GM and its enzyme products can influence the bioavailability, efficacy and toxicity of drugs through direct and indirect mechanisms [6]. This discipline could be the key to precision medicine, especially in the treatment of ARDs by predicting therapeutic responses and optimising treatment through modulation of the microbiota [7]. The chronic course of ARDs requires immunosuppressive (IS) therapy over long periods of time, usually administered orally with gastrointestinal absorption and subsequent renal or hepatic elimination. If there is an interaction between IS therapy and IM during drug absorption, this could offer new possibilities for more efficient and safer patient management, reducing side effects and improving clinical response [7].
Objectives: To collect and analyse studies evaluating the interaction between the human microbiota (HM) and IS treatments for ARDs, and their impact on the disease; with the exception of spondyloarthropathies for which more extensive knowledge is available [8].
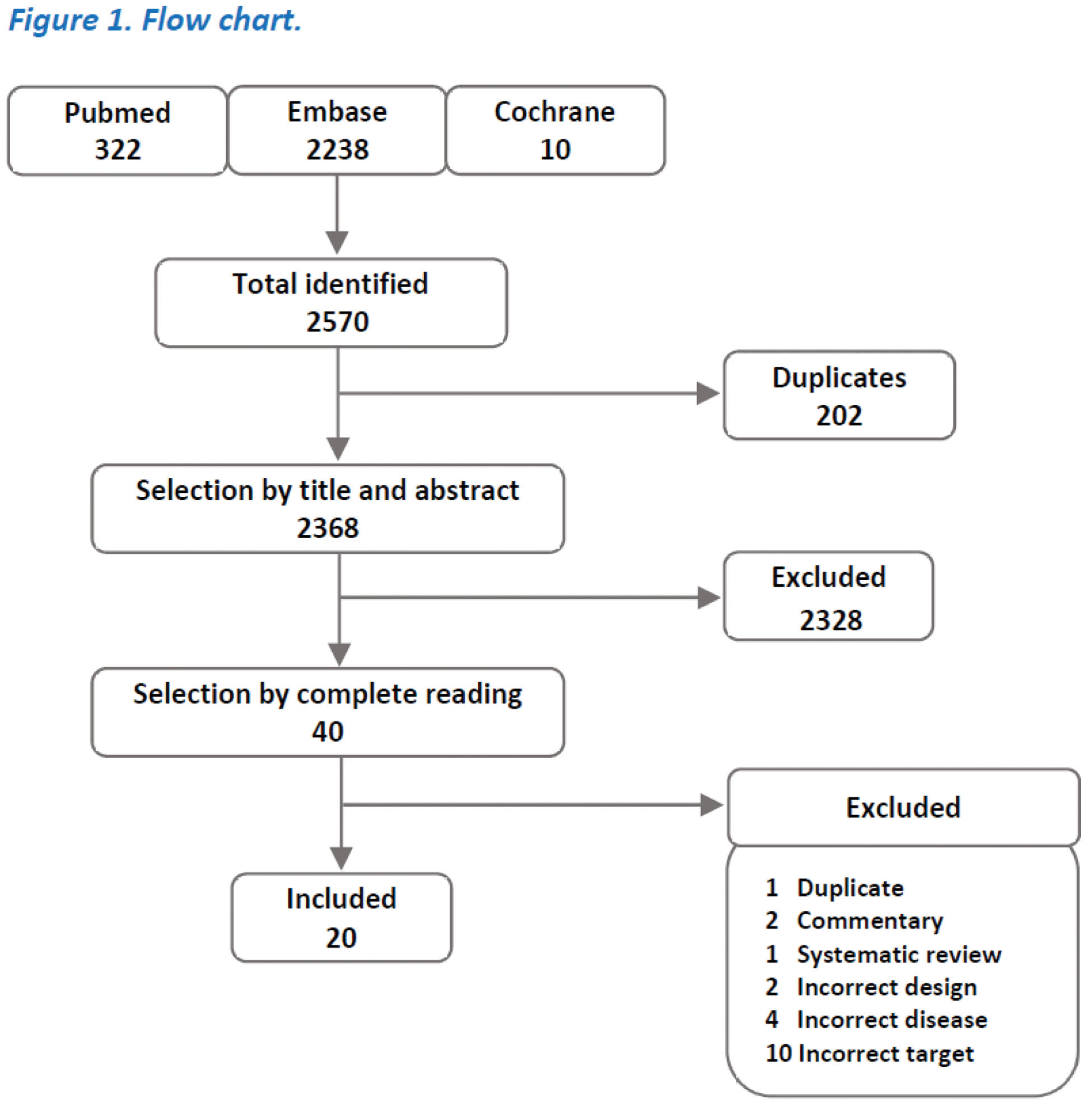
Methods: A systematic review was performed based on an electronic search strategy in PubMed, Embase, and Cochrane Library (inception-02/2024). We included papers studying the interaction of HM and IS treatments in adult patients with ARDs in which parameters of diversity and taxonomic composition were measured. Studies of any language were allowed, prioritising clinical trials but also including prospective and retrospective observational and case-control studies (see Figure 1).
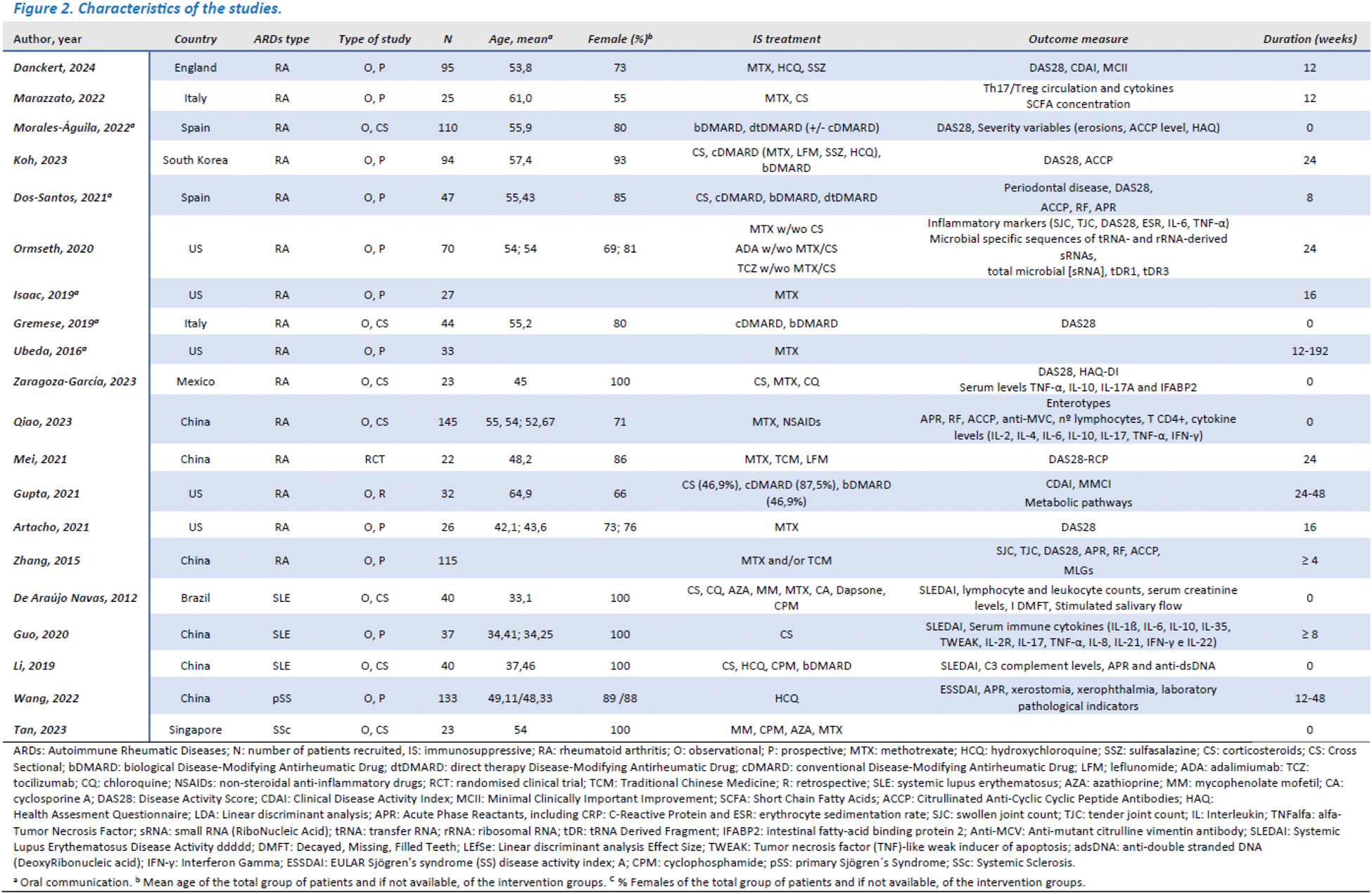
Results: Of 2570 papers identified, 20 were included (15 from rheumatoid arthritis (RA), 3 from systemic lupus erythematosus (SLE), 1 from primary Sjögren’s syndrome (pSS) and 1 from systemic sclerosis (SS)). The paucity of studies and niche specificity limited the study to the gut microbiota. A trend towards decreased diversity and compositional changes in gut microbiota (GM) and partial restitution in patients responding to IS treatment was identified. The studies showed a moderate risk of bias but marked heterogeneity in their development precluded further meta-analysis (see Figure 2).
Conclusion: Changes in HM could be related to the response to IS treatments in ARDs, although there is still too much heterogeneity in the approach of studies to this association, making it difficult to draw firm conclusions. Standardisation of HM studies is necessary to establish comparisons between them.
REFERENCES: [1] Heras A de las. ENFERMEDADES AUTOINMUNES SISTÉMICAS (EAS) [Internet]. Inforeuma. 2024. Available from:
[2] Bellando-Randone S, Russo E, Venerito V, Matucci-Cerinic M, Iannone F. Exploring the Oral Microbiome in Rheumatic Diseases, State of Art and Future Prospective in Personalized Medicine with an AI Approach. Journal of Personalized Medicine. 2021 Jun 30;11(7):625.
[3] Kamada N, Seo SU, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nature Reviews Immunology [Internet]. 2013 Apr 25;13(5):321–35. Available from:
[4] Alkhulaifi MM, Bin A, Alquwayzani MA, Alabdullatif NA, Menezes GA. Gut Microbial Profile Differences in Autoimmune Diseases. Journal of Pure and Applied Microbiology. 2023 Nov 29;17(4):1956–67.
[5] Li H, He J, Jia W. The influence of gut microbiota on drug metabolism and toxicity. Expert Opinion on Drug Metabolism & Toxicology. 2015 Dec 10;12(1):31–40.
[6] Yan H, Su R, Sue H, Sao C, Li X. Pharmacomicrobiology of Methotrexate in Rheumatoid Arthritis: Gut Microbiome as Predictor of Therapeutic Response. Front Immunol. 2021 Dec;12.
[7] Scher JU, Nayak RR, Ubeda C, Turnbaugh PJ, Abramson SB. Pharmacomicrobiomics in inflammatory arthritis: gut microbiome as modulator of therapeutic response. Nat Rev Rheumatol. 2020 May;16(5):282–92.
[8] Lai Y, Tang W, Luo X, Zheng H, Zhang Y, et al. Gut microbiome and metabolome to discover pathogenic bacteria and probiotics in ankylosing spondylitis. Front Immunol. 2024 Apr 22;15:1369116.
Acknowledgements: To my co-authors for their constant and dedicated work, and to the people who support us.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (