

Background: Efgartigimod is an IgG1 antibody Fc fragment that has been engineered for increased affinity to the neonatal Fc receptor (FcRn), and is uniquely composed of only the part of the IgG antibody that naturally binds FcRn. Efgartigimod selectively reduces IgG by blocking FcRn-mediated IgG recycling without impacting antibody production, albumin levels, or other parts of the immune system. Efgartigimod PH20 subcutaneous (SC) is a coformulation that includes efgartigimod and recombinant human hyaluronidase PH20 (rHuPH20). Hyaluronidase is an enzyme that temporarily degrades hyaluronan in the SC space, which reduces the barrier to bulk fluid flow. The role of hyaluronidase in this formulation is to facilitate the SC administration of larger volume within 30 to 90 seconds per injection.
Objectives: To assess the safety profile of efgartigimod PH20 SC across generalized myasthenia gravis (gMG) and chronic inflammatory demyelinating polyneuropathy (CIDP).
Methods: Efgartigimod PH20 SC was assessed using cyclical dosing (4 once-weekly injections) in gMG (ADAPT-SC noninferiority study [SC vs intravenous efgartigimod; NCT04735432] and ongoing open-label extension ADAPT-SC+ trial [NCT04818671; data cut-off: 19 June 2023]) and weekly continuous dosing in CIDP (ADHERE [NCT04281472; stage A: open-label; stage B: placebo-controlled] and ongoing open-label extension ADHERE+ trial [NCT04280718; data cut-off: 15 June 2023]).
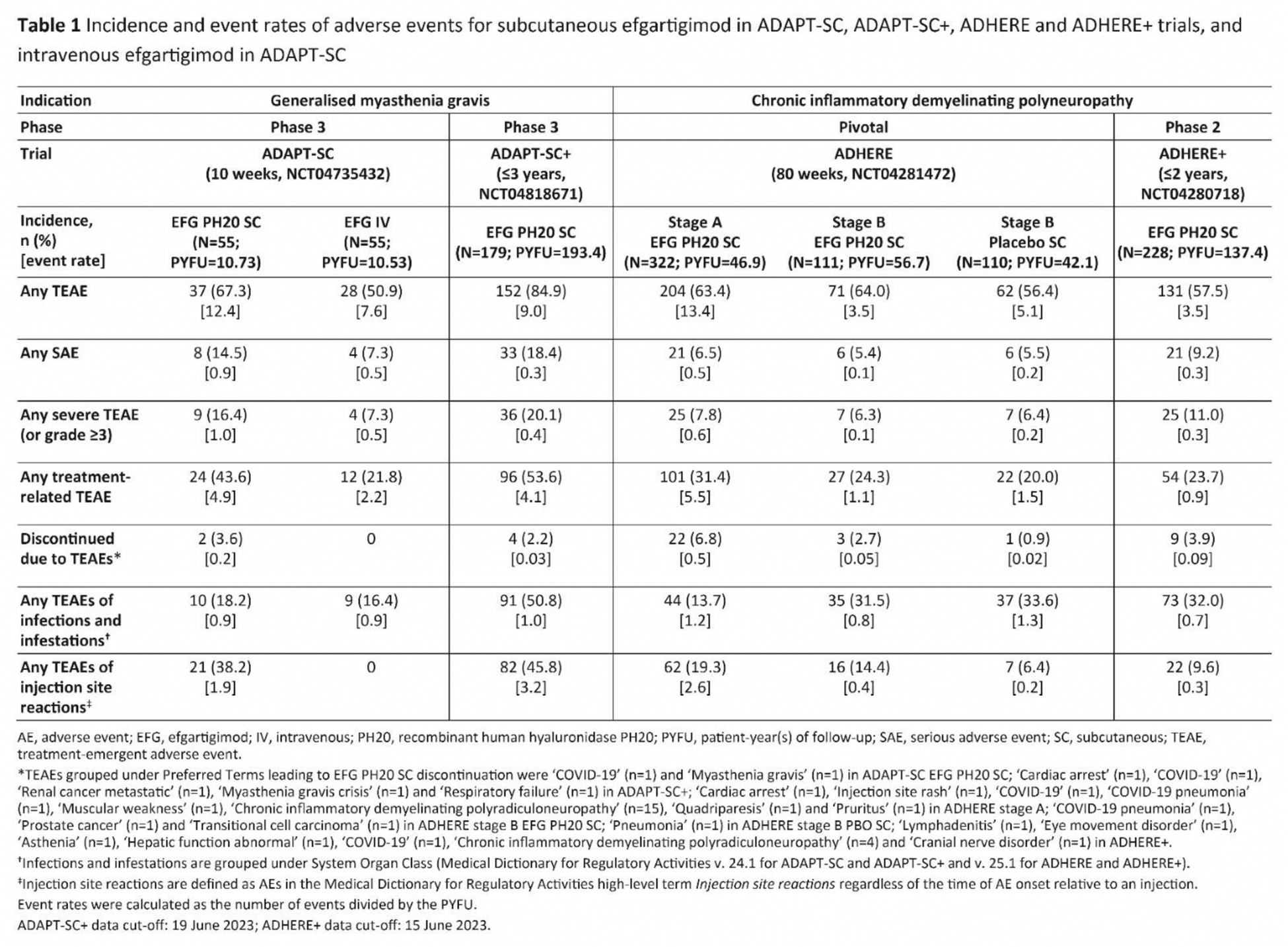
Results: Efgartigimod PH20 SC was well-tolerated and demonstrated a consistent safety profile across indications. Generally, similar rates of treatment-emergent adverse events (TEAEs) between efgartigimod and placebo were observed in ADHERE. A higher rate of injection site reactions (ISRs) was observed with efgartigimod PH20 SC versus placebo in ADHERE (Table 1). Most TEAEs were mild to moderate in severity across studies. There was no increase in TEAE rates, including infections, with repeated treatment. Event rates for AEs did not increase with exposure for MG or CIDP. Discontinuation rates due to TEAEs were consistently low, ranging from 0–6.8% across studies, and were similar to placebo. Discontinuations did not increase with longer exposure. ISRs were mild to moderate in severity, and only 1 participant (in CIDP studies) discontinued efgartigimod PH20 SC because of an ISR (rash at injection site). Efgartigimod PH20 SC did not reduce albumin or increase lipid levels.
Conclusion: Efgartigimod PH20 SC was well-tolerated across indications and dosing regimens. Most TEAEs were mild to moderate in severity and did not increase in frequency with recurrent dosing.
REFERENCES: NIL.
Acknowledgements: Medical writing support was provided by Envision Pharma Group and was funded by argenx.
Disclosure of Interests: Pieter A. van Doorn Annexon Biosciences, argenx, Grifols, Hansa Biopharma, Immunic Therapeutics, Prinses Beatrix Spierfonds, Octapharma, Roche, Sanofi, Sanquin, Thomas Skripuletz Alexion, Alnylam Pharmaceuticals, argenx, Bayer Vital, Biogen, Bristol Myers Squibb, Celgene, Centogene, CSL Behring, Euroimmun, Grifols, Hexal AG, Horizon, Janssen-Cilag, Merck Serono, Novartis, Pfizer, Roche, Sanofi, Siemens, Swedish Orphan Biovitrum, Teva, Viatris, Kelly Gwathmey Alexion, Amgen, argenx, UCB Pharma, Xeris Pharmaceuticals, Jan De Bleecker Alexion, Alnylam, argenx, CSL Behring, Roche, Sanofi Genzyme, UCB, James F. Howard, Jr. Academic CME, Ad Scientiam, Alexion AstraZeneca Rare Disease, Amgen, argenx, Biohaven Ltd, Biologix, Cartesian Therapeutics, Centers for Disease Control and Prevention, CheckRare CME, F. Hoffmann-LaRoche Ltd, Medscape CME, Merck EMB Serono, MGFA, Muscular Dystrophy Association, NIH, NMD Pharma, Novartis, PCORI, PeerView CME, Physicians’ Education Resource (PER) CME, PlatformQ CME, Regeneron Pharmaceuticals, Sanofi US, Toleranzia AB, UCB, Zai Lab, Tuan Vu Alexion, Amgen, argenx, Cartesian Therapeutics, Dianthus, ImmunAbs, Immunovant, Johnson & Johnson, Regeneron, Sanofi, UCB, Jeffrey A. Allen Akcea Therapeutics, Alexion, Alnylam, Annexon, argenx SE, CSL Behring, Grifols, Immuovant, ImmuPharma, Johnson & Johnson, Pfizer, Takeda, Sofiane Agha argenx, Ming Jiang argenx, William Reiss argenx, Peter Ulrichts argenx, Jeff Guptill argenx, Jana Podhorna argenx, Li Liu argenx, Richard A. Lewis Alexion, Annexon Biosciences, argenx, Boehringer Ingelheim, CSL Behring, Dianthus, GBS/CIDP Foundation International, Grifols, Immunovant, Johnson & Johnson, Medscape, Nervosave, Novartis, Nuvig, Peripheral Nerve Society, Sanofi, Seismic, Takeda.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (