

Background: The combination of biological disease-modifying antirheumatic drugs (bDMARDs) and targeted synthetic DMARDs (tsDMARDs) has emerged as a promising strategy to enhance clinical outcomes in patients with immune-mediated diseases (IMIDs), particularly in refractory or complex cases, as some may benefit from targeting multiple inflammatory pathways simultaneously. However, this approach presents significant challenges regarding safety, adverse effect profiles, and the optimization of specific drug combinations.
Objectives: 1) To describe the clinical characteristics of patients with IMIDs receiving dual targeted therapy (DTT) with two bDMARDs or with one bDMARD combined with one tsDMARD.
2) To identify the most used drugs and the main adverse effects of their combination.
3) To evaluate the clinical response to combined therapy.
Methods: This is a retrospective observational study including patients with one or more IMIDs who received combined therapy with bDMARDs and/or tsDMARDs in the Hospital Universitario La Paz from October 2019 to December 2024. Clinical characteristics, bDMARDs and tsDMARDs used, duration of treatment, reasons for discontinuation, disease activity according to indices (DAS28, ASDAS and DAPSA) at baseline and at discontinuation of treatment or at the end of the study observation period if treatment was maintained.
Results: A total of 18 patients with 27 DTT combinations were included. The majority were women (66.7%, 12/18), with a mean age at the start of DTT of 44.2±18.9 years. Among the 18 patients, 7 had spondyloarthritis (SpA, 38.9%), 4 rheumatoid arthritis (RA, 22.2%), 3 psoriatic arthritis (PsA, 16.7%), and 1 juvenile idiopathic arthritis (JIA, 5.6%). The remaining 3 were paediatric patients diagnosed with polyarthritis of possible genetic cause (2) and possible Behçet syndrome (1), with a mean age at the start of DTT of 12.1±2.3 years. The 77.8% of patients associated extra-musculoskeletal manifestations, the most frequent being inflammatory bowel disease (IBD, 38.9%), and 3 patients associated a second IMID subsidiary of treatment with bDMARD/tsDMARD (2 chronic urticaria and 1 chronic idiopathic angioedema). All patients had previously received bDMARD and/or tsDMARD, with a median of 5 prior drugs. Overall, 12 patients (67%) had received only one DTT combination and 6 patients (33%) had received at least one more previous DTT combination (4 patients with 1 prior DTT cycles, 1 with 2 prior DTT cycles, and 1 patient with 3 prior cycles). Concomitantly, 50.0% received conventional DMARDs (cDMARDs) and 33.3% oral glucocorticoids. The primary reason for initiation of DTT cycles was active musculoskeletal (MSK) disease (63%, 17/27), followed in decreasing order by active IBD (11.1%, 3/27), active MSK and IBD disease (7.4%, 2/27), psoriasis (7.4%, 2/27), flares of chronic angioedema (7.4%, 2/27) and neurological inflammatory symptoms (3.7%, 1/27). The most used combinations among the 27 registered DTT cycles were TNF inhibitor (TNFi)+JAK inhibitor (JAKi, 33.3%, 9/27), TNFi+vedolizumab (25.9%, 7/27) and TNFi+anti-IL 23 (11.1%, 3/27). Other combinations were JAKi+vedolizumab, TNFi+anti-IgE, TNFi+anti-IL 12 y 23, anti-IL23+JAKi, anti-IL23+anti-IgE and rituximab+anti-IgE. The median (± interquartile range) time of exposure to DTT was 10.3±59.6 months, with a minimum of 0.7 and a maximum of 60.3 months. The most common side effects during DTT were infections (22.2%, 6/27) and hypertransaminasemia (11.1%, 3/27). The 38.95% (7/18) of patients discontinued the last DTT cycle due to inefficacy (4), infections (2) and loss of follow-up (1). The response to treatment was evaluated with disease activity indices and reviewing the clinical reports of the follow-up visits (see Table 1), finding clinical improvement in 10 patients (55.6%).
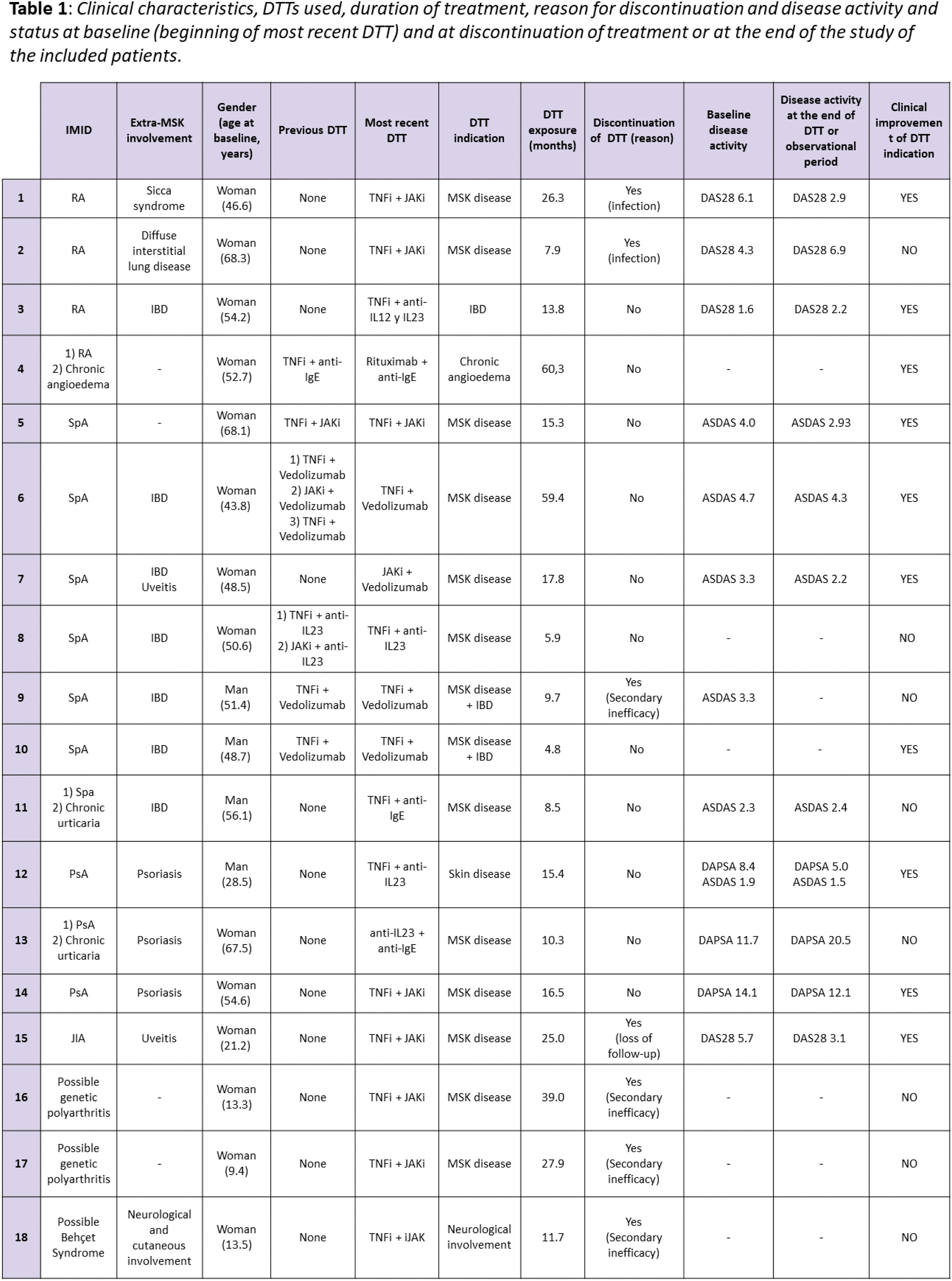
Conclusion: DTT is a viable strategy for patients with IMIDs refractory to previous therapies, showing clinical improvement in more than half of the cases. However, the high incidence of adverse effects and the discontinuation rate highlight the need for close monitoring and careful patient selection. These findings suggest that DTT may be a valid strategy in difficult-to-manage patients, although further studies are required to optimize its use and risk management.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Natalia López-Juanes: None declared, Carlota Ureta: None declared, Marta Novella-Navarro Abbvie, UCB Pharma, Galapagos and Lilly, Irene Monjo-Henry Roche, Novartis, UCB, Janssen, Galapagos, Biopharma and Gedeon Richter, UCB and Roche, Diana Peiteado UCB, Abbvie, Novartis, Lilly, Roche, Janssen and MSD, UCB, Abbvie, Lilly, MSD and Roche, Alejandro Villalba: None declared, Laura Nuño: None declared, María Sanz-Jardon: None declared, Mª Ángeles González-Fernández: None declared, Cristina Suárez: None declared, Agustin Remesal: None declared, Rosa Alcobendas: None declared, Clara Udaondo: None declared, Elena Sendagorta: None declared, Victoria Navarro-Compán AbbVie, Eli Lilly, Fresenius Kabi, Janssen, MSD, Novartis, Pfizer, UCB Pharma, AbbVie, Alfasigma, Eli Lilly, Galapagos, MoonLake, MSD, Novartis, Pfizer, UCB Pharma, AbbVie, Novartis, Alejandro Balsa: None declared, Eugenio De Miguel Novartis, Abbvie, Pfizer, Janssen, Lilly, AbbVie, Novartis, Pfizer, MSD, BMS, UCB, Roche, Grunental, Janssen, Sanofi, Lylly, Abbvie, Novartis, Pfizer, Roche, Chamaida Plasencia-Rodríguez: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (