

Background: Physical activity is a key component in the management of axial spondyloarthritis (axSpA), with evidence indicating that regular exercise can reduce symptoms and enhance overall functional capacity.
Objectives: This study aims to identify the factors associated with physical activity engagement in patients with axSpA.
Methods: IMAS is a cross-sectional online survey conducted from 2017 to 2022, of 5,557 unselected patients with axial spondyloarthritis (axSpA) across Europe, North America, Latin America, Asia, and South Africa. The sample was categorized into two groups according to the following question “Do you do any physical or sporting activity?”: patients who reported that they engaged in physical activity and those who did not. The independent variables evaluated included sociodemographic factors (age, gender, and patient organizations membership), disease characteristics (diagnostic delay, HLA-B27, uveitis, inflammatory bowel disease, and psoriasis), patient-reported outcomes (disease activity [BASDAI 0-10], spinal stiffness [3-12], functional limitation [0-54], and mental health [GHQ-12]), mental comorbidities (anxiety, depression, and sleep disorders), and previous treatments (non-steroidal anti-inflammatory drugs [NSAIDs], conventional synthetic disease-modifying antirheumatic drugs [csDMARDs], and biologic disease-modifying antirheumatic drugs [bDMARDs]). Mann-Whitney tests, chi-square tests, and logistic regression analyses assessed the relationships between these factors and physical activity among axSpA patients.
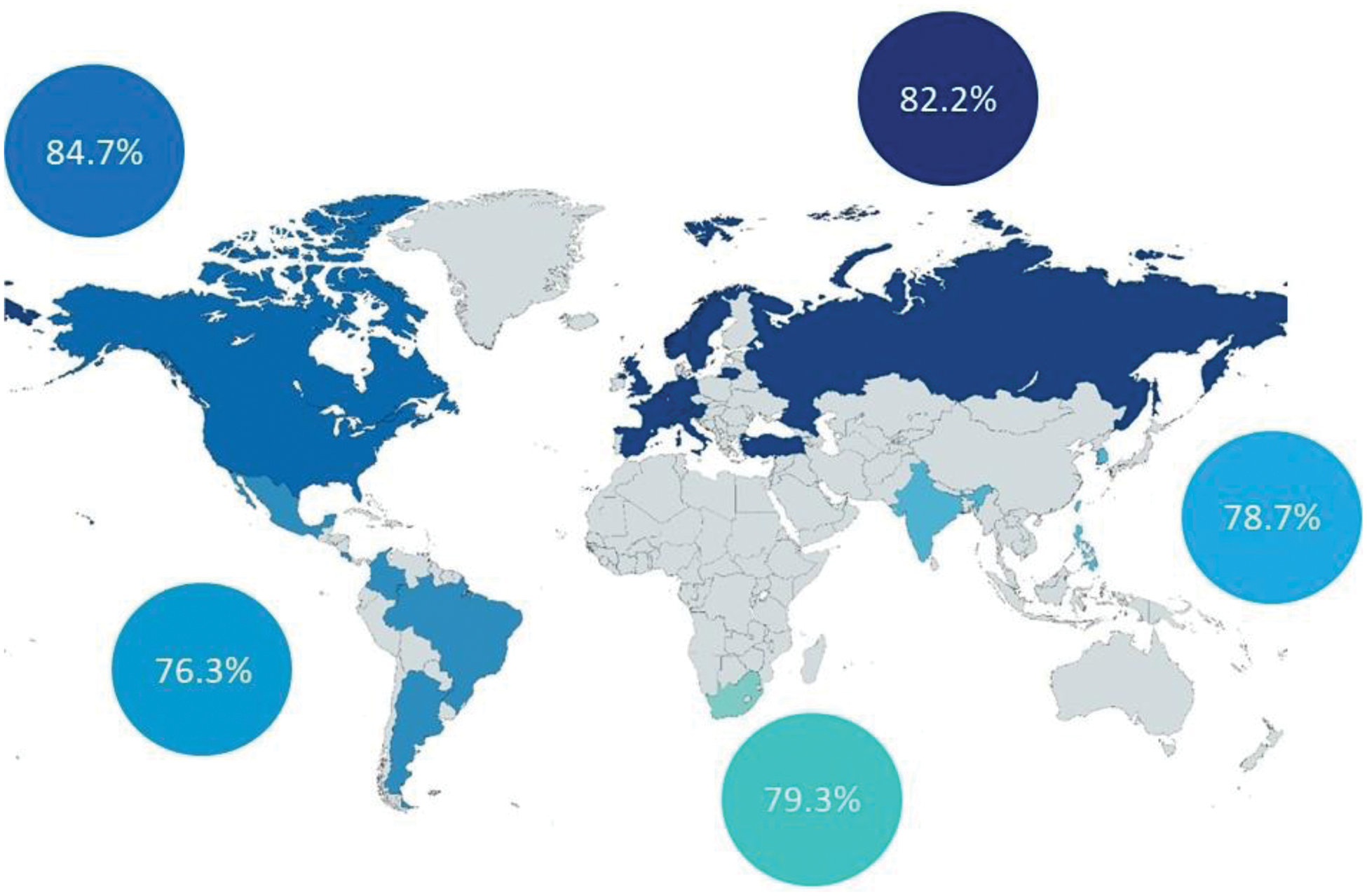
Results: Among the 5476 participants who responded to the question on physical activity, 81.6% reported engaging in physical activity. This proportion was higher in North America (84.7%) and Europe (82.2%), and lowest in South Africa (79.3%), Asia (78.7%) and Latin America (76.3%; Figure 1). Patients who engaged in physical activity were more likely to be members of patient organizations, have a longer diagnostic delay, exhibit a higher prevalence of uveitis, report lower disease activity, experience less spinal stiffness, have less functional limitation, and report better mental health. Additionally, the patients had a lower anxiety score. In the multivariate logistic regression analysis, factors significantly associated with engagement in physical activity included membership in a patient organization (OR = 1.41), presence of uveitis (OR = 1.36), lower disease activity (OR = 0.95), reduced spinal stiffness (OR = 0.95), fewer functional limitations (OR = 0.99), and better mental health (OR= 0.98; Table 1).
Conclusion: 8 out of 10 patients with axial spondyloarthritis (axSpA) engage in physical activity, with slight regional variations observed. Patients engaged in physical activity reported lower disease activity, reduced spinal stiffness, fewer functional limitations, and better mental health. Furthermore, membership of patient organizations was associated with higher levels of physical activity. Although causal relationships cannot be established from this study, the findings underscore the importance of promoting physical activity, particularly among those least active, given the positive impact on patient-reported outcomes. These results suggest that physical activity should be considered as a key component of disease management.
REFERENCES: NIL.
Physical activity by region (N= 5,557)
Logistic regression analysis of factors associated with physical activity (N= 4,548)
| Univariable logistic regression | Multivariable logistic regression | |||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Member of patient organization | 1.40 | 1.22, 1.61 | 1.41 | 1.20, 1.66 |
| Diagnostic delay | 1.01 | 0.99, 1.02 | - | - |
| Uveitis. Yes | 1.44 | 1.20, 1.73 | 1.36 | 1.11, 1.65 |
| BASDAI (0-10 ) | 0.89 | 0.85, 0.92 | 0.95 | 0.90, 0.99 |
| Spinal stiffness (3-12 ) | 0.92 | 0.89, 0.95 | 0.95 | 0.92, 0.99 |
| Functional limitation (0-54 ) | 0.99 | 0.98, 0.99 | 0.99 | 0.98, 0.99 |
| GHQ-12 (0-12 ) | 0.95 | 0.93, 0.96 | 0.98 | 0.96, 0.99 |
| Anxiety. Yes | 0.83 | 0.71, 0.96 | 0.86 | 0.73. 1.03 |
Acknowledgements: This study was supported by Novartis Pharma AG. As of 2 January 2024, IMAS is owned solely by ASIF, following an in-kind donation of the project by Novartis Pharma AG. The authors would like to thank all patients who participated in the study.
Disclosure of Interests: Marco Garrido-Cumbrera Novartis, Fernando Sommerfleck Abbvie, Eli Lilly, Janssen, Novartis, Abbvie, Novartis, Janssen, Denis Poddubnyy AbbVie, BMS, Celgene, Janssen, Lilly, MSD, Novartis, Pfizer, Roche and UCB, AbbVie, MSD, Novartis, and Pfizer, José Correa-Fernández: None declared, Jo Lowe No personal funding, but ASIF has received funding from Novartis, UCB, Lilly, Abbvie, Boehringer Ingleheim, Pfizer, Janssen, Christine Bundy AbbVie, Amgen, Celgene, Janssen, Lilly, Novartis and Pfizer, Souzi Makri Novartis, GSK and Bayer, Shashank Murlidhar Akerkar Pfizer, Novartis, Eli Lilly, Jansen, Victoria Navarro-Compán AbbVie, Eli Lilly, Fresenius Kabi, Janssen, MSD, Novartis, Pfizer, UCB Pharma, AbbVie, Alfasigma, Eli Lilly, Galapagos, MoonLake, MSD, Novartis, Pfizer, UCB Pharma, AbbVie, Novartis.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (