

Background: Although most European countries apply the EULAR recommendation to guide treatment of newly diagnosed rheumatoid arthritis (RA), the extent to which country-specific or regional variations in application of these guidelines impact the pattern of treatment sequences of disease-modifying antirheumatic drugs (DMARDs) remains poorly understood.
Objectives: To describe the choice, type (mono- vs. combination-therapy) and sequencing of the first DMARDs used in newly diagnosed RA, and to understand the characteristics of patients at start of each new DMARD.
Methods: We used clinical and treatment-related data from three centers: Region Stockholm (Sweden, SW), Leiden (Netherlands, NL), and Oslo (Norway, NO) on patients newly diagnosed with RA who started their first ever DMARD from 2008-2023 (SW), 2011-2024 (NL), 2001-2012 (NO, follow-up ending in 2024). The type of DMARD initiated was categorised as MTX, other conventional synthetic DMARD (csDMARD: hydroxychloroquine, sulfasalazine, leflunomide, cyclosporine, azathioprine), tumor necrosis factor inhibitors (TNFi: adalimumab, infliximab, etanercept, certolizumab pegol, golimumab), non-TNFi (abatacept, anakinra, rituximab, tocilizumab, sarilumab), and Janus Kinase inhibitors (JAKi: tofacitinib, baricitinib, upadacitib, filgotinib). If a patient started two or more DMARD in combination, the patient was included in the respective type according to the following hierarchy: 1) TNFi, 2) non-TNFi, 3) JAKi, 4) MTX, and 5) other csDMARD. Combination therapy was defined as start of two DMARDs within 30 days without discontinuation of the first one. Thus, a patient starting e.g., a TNFi and MTX in combination was assigned to the “TNFi” category and marked as a “combination therapy”. Duration of treatment was defined as the time from the start of the DMARD and the earliest between the stop of the treatment and the start of the following line of treatment DMARD, and reported as median. Disease characteristics of patients at start of each line of DMARD were reported as mean and standard deviations.
Results: A total of 7415 RA patients (3850 SW, 1671 NL, 1894 NO) starting a first ever DMARD were included. MTX was the most common first DMARD across the three centers (88% SW, 80% NL, 80% NO). The use of other csDMARDs as first-line therapy was less common (6% SW, 15% NL, 17% NO), Table 1 and Figure 1. Few patients (<5%) started their first DMARD as combination therapy, but 59% (SW), 37% (NL) and 10% (NO) added an additional DMARD to the first beyond the first 30 days of initiation of the first DMARD. 51% (SW), 65% (NL) and 36% (NO) switched to a second DMARD, and 26% (SW), 43% (NL) and 17% (NO) started a third DMARD during the study period. While starting a TNFi as second or third DMARD was common in SE (46%) and NO (56%), in NL a higher proportion of patients switched or added another csDMARD (63%). The treatment pattern over time (Figure 1) among those still on treatment at each time point, was more similar between SW and NO: a steady increase in the use of bDMARDs while in NL the initiation of bDMARDs was lower but the continued use of MTX and other csDMARDs was higher.
Disease characteristics and type of DMARD started for the first 3 treatment DMARDs used in newly diagnosed patients with RA.
| Sweden | Netherlands | Norway | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Line of DMARD | First | Second | Third | First | Second | Third | First | Second | Third |
| N | 3850 | 1952 | 990 | 1671 | 1086 | 718 | 1894 | 678 | 320 |
| MTX | 3371 | 263 | 143 | 1343 | 324 | 193 | 1506 | 141 | 24 |
| Other csDMARD | 242 | 617 | 172 | 257 | 682 | 390 | 315 | 128 | 37 |
| TNFi | 154 | 893 | 497 | 55 | 57 | 111 | 72 | 377 | 213 |
| Non TNFi | 78 | 144 | 137 | 12 | 20 | 15 | <5 | 30 | 44 |
| JAKi | 5 | 35 | 41 | <5 | <5 | 9 | 0 | <5 | <5 |
| Combo therapy at treatment start, n(% ) | 194 (5) | 64 (3) | 24 (2) | 80 (5) | 133 (12) | 64 (9) | 0 | 0 | 0 |
| Added to previous treatment, n(% ) | - | 1148 (59) | 274 (28) | - | 404 (37) | 278 (39) | - | 69 (10) | 35 (11) |
| Female, n(% ) | 2646 (69) | 1448 (74) | 764 (77) | 1094 (65) | 721 (66) | 505 (70) | 1264 (67) | 486 (72) | 239 (75) |
| Age, mean(SD ) | 60 (16) | 55 (16) | 52 (15) | 61 (15) | 59 (15) | 57 (14) | 55 (14) | 50 (14) | 47 (13) |
| Disease Characteristics at treatment initiation | |||||||||
| 28 swollen joint count, mean(SD ) | 8 (6) | 8 (5) | 7 (5) | 5 (5) | 5 (5) | 6 (5) | 7 (6) | 7 (6) | 7 (6) |
| 28 tender joint count, mean(SD ) | 8 (6) | 8 (6) | 8 (6) | 6 (5) | 6 (5) | 6 (5) | 8 (7) | 8 (7) | 9 (7) |
| ESR, mean(SD ) | 35 (25) | 34 (25) | 33 (25) | 32 (27) | 34 (29) | 35 (30) | 28 (22) | 29 (22) | 30 (23) |
| CRP, mean(SD ) | 21 (29) | 20 (29) | 20 (31) | 20 (32) | 21 (33) | 25 (38) | 22 (29) | 23 (28) | 23 (27) |
| Pain, VAS scale, mean(SD ) | 55 (26) | 57 (26) | 59 (25) | - | - | - | 45 (25) | 47 (25) | 49 (24) |
| Patient global, VAS scale, mean(SD ) | 51 (27) | 53 (26) | 56 (26) | 54 (23) | 56 (23) | 58 (23) | - | - | - |
Treatment pattern across the included centers in Sweden (SW), Netherlands (NL), and Norway (NO). The three upper panel represents the treatment allocation from start of first DMARD and progressively every 6 months, while the bottom panel represents the proportion of treatment across DMARD switches.
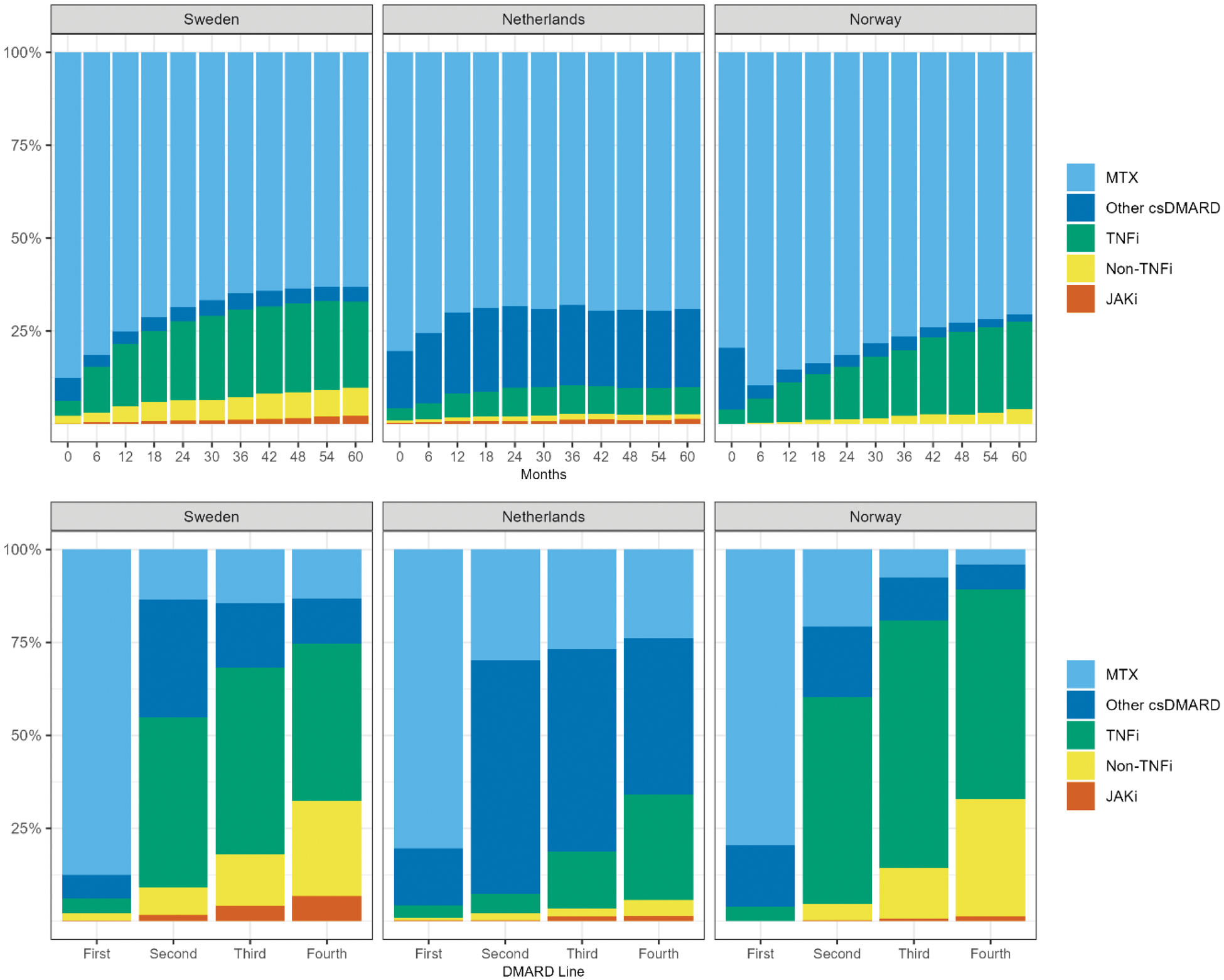
Conclusion: Despite similar treatment guidelines within the Europe countries, there are clear differences in the use and sequencing of DMARDs in newly diagnosed RA, particularly for second-line DMARDs. Further analyses are needed to understand how these differences affect remission rates and other treatment outcomes in RA.
REFERENCES: NIL.
Acknowledgements: This project has received funding from Horizon Europe programme under grant agreement no. 101095052 (SQUEEZE) and no. 101080711 (SPIDERR). We thank David Steeman for his help in extracting and processing the electronic health record data.
Disclosure of Interests: Daniela Di Giuseppe: None declared, Nils Steinz: None declared, Joe Sexton: None declared, Claudia Anna Hana: None declared, Helga Lechner-Radner: None declared, Daniela Sieghart: None declared, Sella Aarrestad Provan: None declared, Rachel Knevel: None declared, Johan Askling agreements between Karolinska Institutet (with JA as PI) and the listed entities, mainly for the national safety monitoring of rheumatology immunomodulators in Sweden (ARTIS): Abbvie, BMS, Eli Lilly, Galapagos, MSD, Pfizer, Roche, Samsung Bioepis, Sanofi;.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (