

Background: Patients with inflammatory rheumatic diseases (IRDs) frequently experience psychological distress alongside physical symptoms, diminishing their quality of life. Digital mental health interventions provide a scalable solution to improve access to psychological support, yet evidence for their effectiveness in IRD populations remains limited.
Objectives: The aim of this trial was to investigate the efficacy of a self-guided web-based cognitive behavioral therapy (CBT) intervention (medical device Vila RaVie ) for individuals with IRDs.
Methods: This decentralized prospective randomized clinical trial (RELIEVE) was completed between February 22, 2024, and August 29, 2024 (DRKS00032862). Participants were recruited across Germany if they were aged 18 years or older, who had been diagnosed with rheumatoid arthritis (RA), psoriatic arthritis (PsA), or systemic lupus erythematosus (SLE) reported psychological distress ([HADS] >8 points on at least one subscale) and demonstrated reduced quality of life ([AQoL-8D] Score ≤ 73.3%). Participants were randomized to either the web-based CBT intervention or a waitlist control condition. The primary outcomes were change in psychological distress (HADS) and quality of life (AQoL-8D) from baseline to three months. Secondary outcomes included changes in self-efficacy (SES6G), health literacy (HLS-EU-Q16), perceived stress (PSS-10), functional impairment (WSAS), depression (PHQ-9) and anxiety (GAD-7).
Results: A total of 102 participants (mean [SD] age, 47.2 [12.9] years; 92 [90.2%] female) were randomized either to the intervention group (n = 53) or the control group (n = 49). 33 (32%) participants had RA, 37 (36%) PsA and 32 (31%) SLE. The intervention group showed a significantly greater reduction in psychological distress after 12 weeks with an effect size of d=0.72 (least-squares mean difference −3.6; SE 1.1; p<0·001; Figure 1) and a greater improvement of quality of life compared to the control group with an effect size of d=0.47 (least-squares mean difference 0.04; SE 0.02; p=0.058), see Table 1. Additionally, significant improvements were observed in self-efficacy (d =0.48; p=0.053), health literacy (d=0.52; p=0.016), perceived stress (d=0.68; p=0.005), depression (d=0.68; p=0.010), and anxiety (d=0.77; p=0.002), favoring the intervention. No significant change was noted in impaired functioning (d=0.10; p=0.665). No adverse events related to the intervention were reported.
Conclusion: This randomized clinical trial provides evidence supporting the effectiveness and safety of a self-guided web-based CBT intervention in reducing psychological distress and enhancing quality of life in individuals with IRDs. These findings suggest that such digital interventions could be a valuable, scalable approach to addressing mental health needs in this population, though further confirmatory studies are warranted.
REFERENCES: NIL.
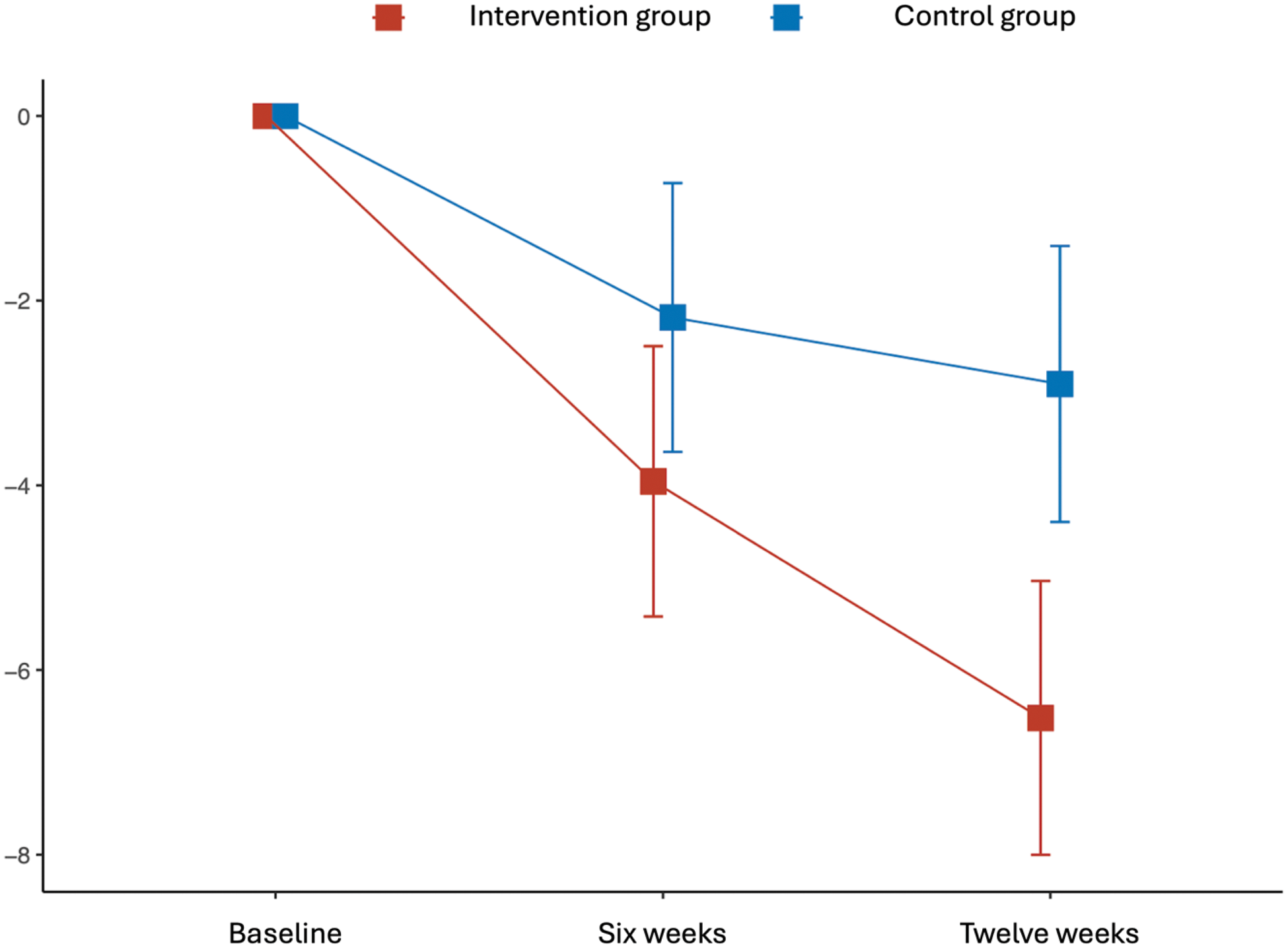
Results of the primary outcomes.
| Timepoint | Intervention group | Control group | Between-arm difference | p value (effect size) | ||
|---|---|---|---|---|---|---|
| Mean (SD) | Least-squares mean
| Mean (SD) | Least-squares mean
| Estimate (SE) | ||
| HADS | ||||||
| Baseline | 19.7 (5.3) | 20.4 (5.7) | ||||
| 6 weeks | 15.8 (5.8) | -4.0 (0.7; -5.4 to -2.5) | 18.1 (7.0) | -2.2 (0.7; -3.6 to -0.7) | -1.8 (1.1) | 0.096 (0.35) |
| 12 weeks | 13.4 (6.0) | -6.5 (0.8; -8.0 to -5.0) | 17.4 (6.7) | -2.9 (0.8; -4.4 to -1.4) | -3.6 (1.1) | 0.001 (0.72) |
| Aqol-8D | ||||||
| Baseline | 0.62 (0.11) | 0.62 (0.11) | ||||
| 6 weeks | 0.66 (0.11) | 0.04 (0.01; 0.02 to 0.07) | 0.65 (0.13) | 0.02 (0.01; -0.01 to 0.04) | 0.03 (0.02) | 0.188 (0.28) |
| 12 weeks | 0.69 (0.13) | 0.07 (0.02; 0.04 to 0.10) | 0.66 (0.14) | 0.03 (0.02; -0.00 to 0.06) | 0.04 (0.02) | 0.058 (0.47) |
Aqol: Assessment of Quality of Life; HADS: Hospital Anxiety and Depression Scale; SD: Standard deviation; SE: Standard error
Least-squares mean change of HADS scores.
Acknowledgements: We thank all patients for their participation in this study, and thank the Deutsche Rheuma-Liga e.V., Lupus Erythematodes Selbsthilfegemeinschaft e.V., Psoriasis-Netz, Deutscher Psoriasis Bund e.V., all other patient support groups, organizations and individuals for their support of this trial.
Disclosure of Interests: Johannes Knitza Abbvie, AstraZeneca, BMS, Boehringer Ingelheim, Chugai, Fraunhofer, Fachverband Rheumatologische Fachassistenz, GAIA, Galapagos, GSK, Janssen, Lilly, Medac, Novartis, Pfizer, Rheumaakademie, Sanofi, Sobi, UCB, Vila Health, GAIA, GSK, Vila Health, Abbvie, GSK, Vila Health, Julia Kraus Vila Health, Vila Health, Martin Krusche Abbvie, Isabell Haase Abbvie, Philipp Klemm Medac, Axel Hueber Abbvie, Alexander Pfeil Abbvie, Ulrich Drott Abbvie, Sebastian Kuhn med.digital, Philipp Klein GAIA.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (