

Background: Guidelines for chronic inflammatory diseases or pain management do not include gender differences, even though differences exist between males and females regarding pain and treatment effect perceptions.
Objectives: Following the Yu-Matter study [1], this post-hoc analysis aimed to assess sex-related differences in the patient’s satisfaction and perception when switching from an adalimumab (reference or biosimilar) to CT-P17 a high concentration citrate-free adalimumab biosimilar (BioS).
Methods: Yu-Matter (NCT05427942), a prospective multicentric observational French study included patients with IBD or CIRD, treated with adalimumab (either the reference product or a BioS with low concentration, 50 mg/mL). Patients were switched to CT-P17 an adalimumab BioS with high concentration and citrate-free and followed-up for 3 months. The main outcome was patient satisfaction with CT-P17 after 3 months (M3), collected through a 7-point Likert scale and analysed as satisfied. Patient beliefs about medicine were assessed before the switch, using the BMQ questionnaire including perceived necessity and concerns subscores, and knowledge was assessed through a health literacy questionnaire (HLS-EU-Q16). Association between sex and the different criteria of the Yu-Matter study was explored through Wilcoxon test, Chi-squared test, or Fisher exact test.
Results: In the analysis population (IBS or CIRD) of 232 patients, 115 were women with a median age of 43 years with interquartile range [30-56]. The median BMI was 24.5 kg/m² [22-27.4] with 28% of patients with a rheumatic disease (15.1% ankylosing spondylarthritis [AS], 7.3% rheumatoid arthritis [RA], 3% axial spondyloarthritis with no signs of AS [nr-axSpA] and 2.6% psoriatic arthritis [PsA]). At baseline, there were no statistical differences between men and women in disease duration (median: 10 years vs. 9 years; p=0.234) and disease activity (17.6% vs. 22.4% with active disease; p=0.375). The type of adalimumab before switch (40.5% were on reference adalimumab 40mg and 19.4% treated with citrate adalimumab 40mg) was similar between both sexes. It was the first switch for 40.1% of patients without any difference between men and women (41% vs. 39%). Regarding patients’ expectations of CT-P17, 55.6% of men and 49.6% of women (p=0.546) expected easier ease of use and more comfort (52.1% vs. 55.7%; p=0.522) with CT-P17 without difference between sexes. Regarding pain at the injection site with CT-P17, 59% of men and 73% of women expected less pain. There was no significant difference also in the overall satisfaction at baseline (81.2% for men vs. 75.7% for women) and at M3 (82.1% of men and 68.7% of women). There was no difference in the evolution of satisfaction between baseline and M3 according to sex (83.8% vs. 77.4%; p=0.22). A similar proportion of men and women received enough information on the treatment (82.1% vs. 83.5%: p=0.774) and the reason for switching (82.9% vs. 77.4%; p=0.292). Pain with the previous adalimumab treatment was higher for women, with a median score of 2 [1-4] vs 3 [1; 5] for men and women, respectively (p=0.004) and similarly it was higher for women after M3 (0 [0-2] vs. 1 [0-3], respectively, p=0.006). Women experienced more itching at the injection site and less absence of hematoma after M3 (median itching score: 0 [0-0] in men vs. 0 [0-2] in women; p<0.001) and no hematoma: 91.5% men vs. 67.4% women; p <0.001). Overall good tolerance was defined as pain and itching scores <4/10, no redness and no hematoma and this was also significantly better in men (72.3% men vs. 43.2% women: p<0.001) ( cf Figure 1). Patients’ beliefs (BMQ subscore) and health literacy (HLS-EU-Q16 score) did not show any significant difference between men and women.
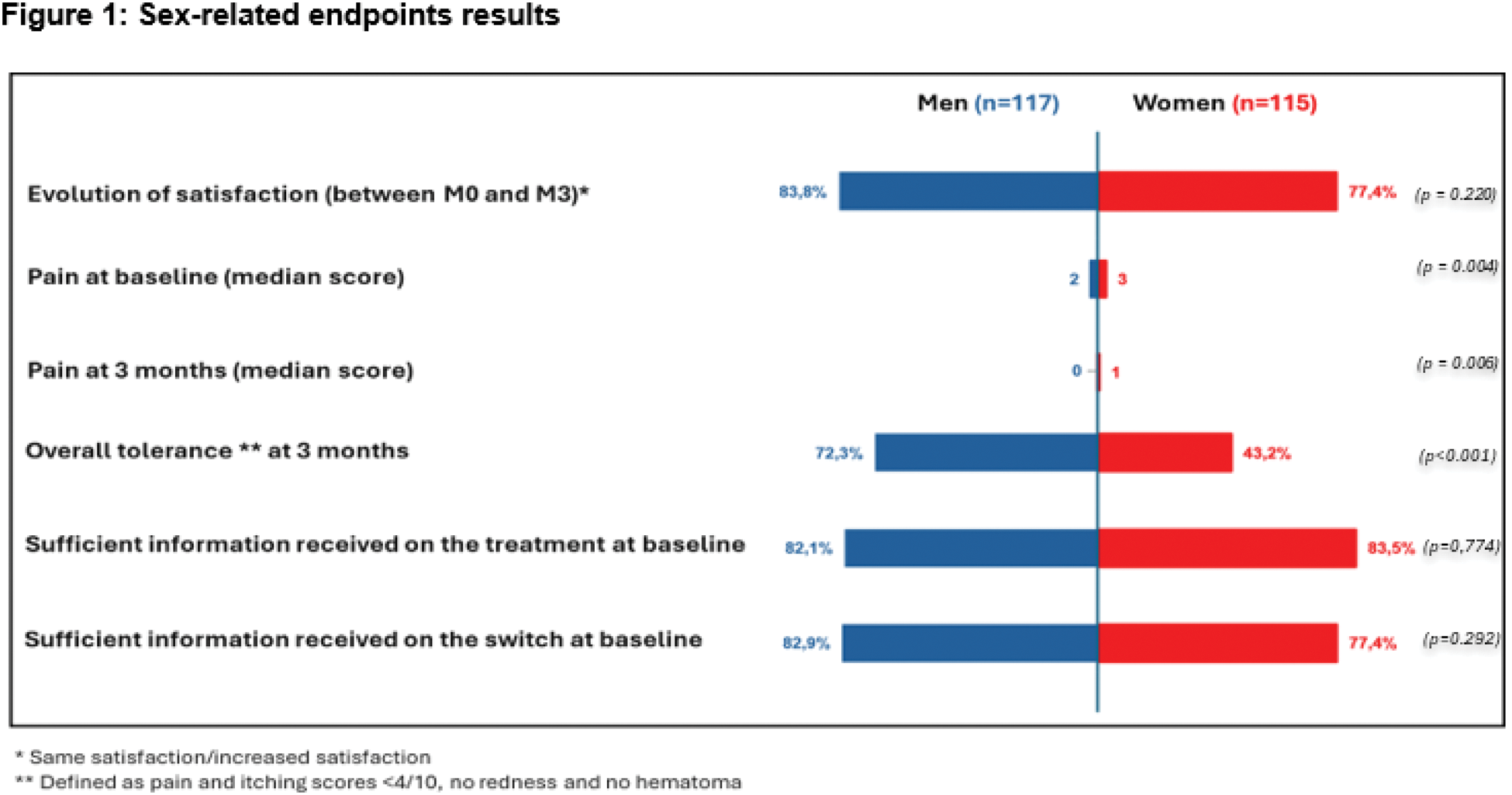
Conclusion: This post-hoc analysis showed that women switching to CT-P17 were globally satisfied; however, women experienced higher pain and lower overall tolerance. This difference should be considered in day-to-day management to help the physician to prevent the nocebo effect in women, and specific studies should focus on this point.
REFERENCES: [1] Bouguen G, Gossec L, Abitbol V, Senbel E, Bonnaud G, Roblin X, Bouhnik Y, Nancey S, Mathieu N, Filippi J, Vuitton L, Nahon S, Dellal A, Denis A, Foulley L, Habauzit C, Benkhalifa S, Marotte H. Patient Satisfaction and Experience with CT-P17 Following Transition from Reference Adalimumab or Another Adalimumab Biosimilar: Results from the Real-World YU-MATTER Study. BioDrugs. 2024 Nov;38(6):867-878. doi: 10.1007/s40259-024-00681-2. Epub 2024 Sep 25. PMID: 39322802; PMCID: PMC11530508.
Acknowledgements: NIL.
Disclosure of Interests: Hubert Marotte personal fees and non-financial support from AbbVie; personal fees from Accord; grants, personal fees and non-financial support from Biogen; grants, personal fees, and non-financial support from Bristol Myers Squibb; personal fees and non-financial support from Cell Trion Healthcare; grants and non-financial support from Fresenius Kabi; personal fees and non-financial support from Galapagos; non-financial support from Janssen; grants personal fees, and non-financial support from Lilly; personal fees and non-financial support from MSD; grants, personal fees, and non-financial support from Nordic Pharma; personal fees, non-financial support and other from Novartis; grants, personal fees and non-financial support from Pfizer; personal fees from Roche Chugai; grants, personal fees, and non-financial support from Sanofi, outside the submitted work, personal fees and non-financial support from AbbVie; personal fees from Accord; grants, personal fees and non-financial support from Biogen; grants, personal fees, and non-financial support from Bristol Myers Squibb; personal fees and non-financial support from Cell Trion Healthcare; grants and non-financial support from Fresenius Kabi; personal fees and non-financial support from Galapagos; non-financial support from Janssen; grants personal fees, and non-financial support from Lilly; personal fees and non-financial support from MSD; grants, personal fees, and non-financial support from Nordic Pharma; personal fees, non-financial support and other from Novartis; grants, personal fees and non-financial support from Pfizer; personal fees from Roche Chugai; grants, personal fees, and non-financial support from Sanofi, outside the submitted work, personal fees and non-financial support from AbbVie; personal fees from Accord; grants, personal fees and non-financial support from Biogen; grants, personal fees, and non-financial support from Bristol Myers Squibb; personal fees and non-financial support from Cell Trion Healthcare; grants and non-financial support from Fresenius Kabi; personal fees and non-financial support from Galapagos; non-financial support from Janssen; grants personal fees, and non-financial support from Lilly; personal fees and non-financial support from MSD; grants, personal fees, and non-financial support from Nordic Pharma; personal fees, non-financial support and other from Novartis; grants, personal fees and non-financial support from Pfizer; personal fees from Roche Chugai; grants, personal fees, and non-financial support from Sanofi, outside the submitted work, Guillaume BOUGUEN Abbvie, Amgen, Biogen, Celltrion, Fresenius Kabi, Galapagos, Gilead, Janssen, Lilly, Pfizer, Takeda, Sandoz, Abbvie, Amgen, Biogen, Celltrion, Fresenius Kabi, Galapagos, Gilead, Janssen, Lilly, Pfizer, Takeda, Sandoz, Abbvie, Amgen, Biogen, Celltrion, Fresenius Kabi, Galapagos, Gilead, Janssen, Lilly, Pfizer, Takeda, Sandoz, Azeddine Dellal Celltrion, Fresenius, Lucile Foulley Celltrion Healthcare France, Caroline HABAUZIT Celltrion Healthcare France, Salim Benkhalifa Celltrion Healthcare France, Laure Gossec AbbVie, Amgen, BMS, Celltrion, Janssen, Lilly, MSD, Novartis, Pfizer, UCB, AbbVie, Biogen, Lilly, Novartis, UCB.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (