

Background: Familial Mediterranean Fever (FMF) is a monogenic autoinflammatory disorder marked by recurrent fever and serositis. While colchicine remains the cornerstone of FMF treatment, some patients are resistant to or intolerant of it. Canakinumab, an interleukin-1β inhibitor, has demonstrated efficacy in treating colchicine-resistant/intolerant FMF. More data is required regarding dose, dosing interval, patient with associated comorbidity along with efficacy and safety.
Objectives: This study aims to assess the efficacy and safety of canakinumab treatment in patients with FMF managed at a tertiary center.
Methods: Patients diagnosed with FMF according to the Tel Hashomer criteria and treated with canakinumab, who were under follow-up at the Hacettepe University Department of Rheumatology, were retrospectively analyzed. Data on demographic characteristics, age at diagnosis, MEFV gene mutation profiles, presence of amyloidosis, comorbid conditions, colchicine dosage at the last visit, canakinumab dosage and intervals, duration of therapy, and reasons for treatment discontinuation were recorded.
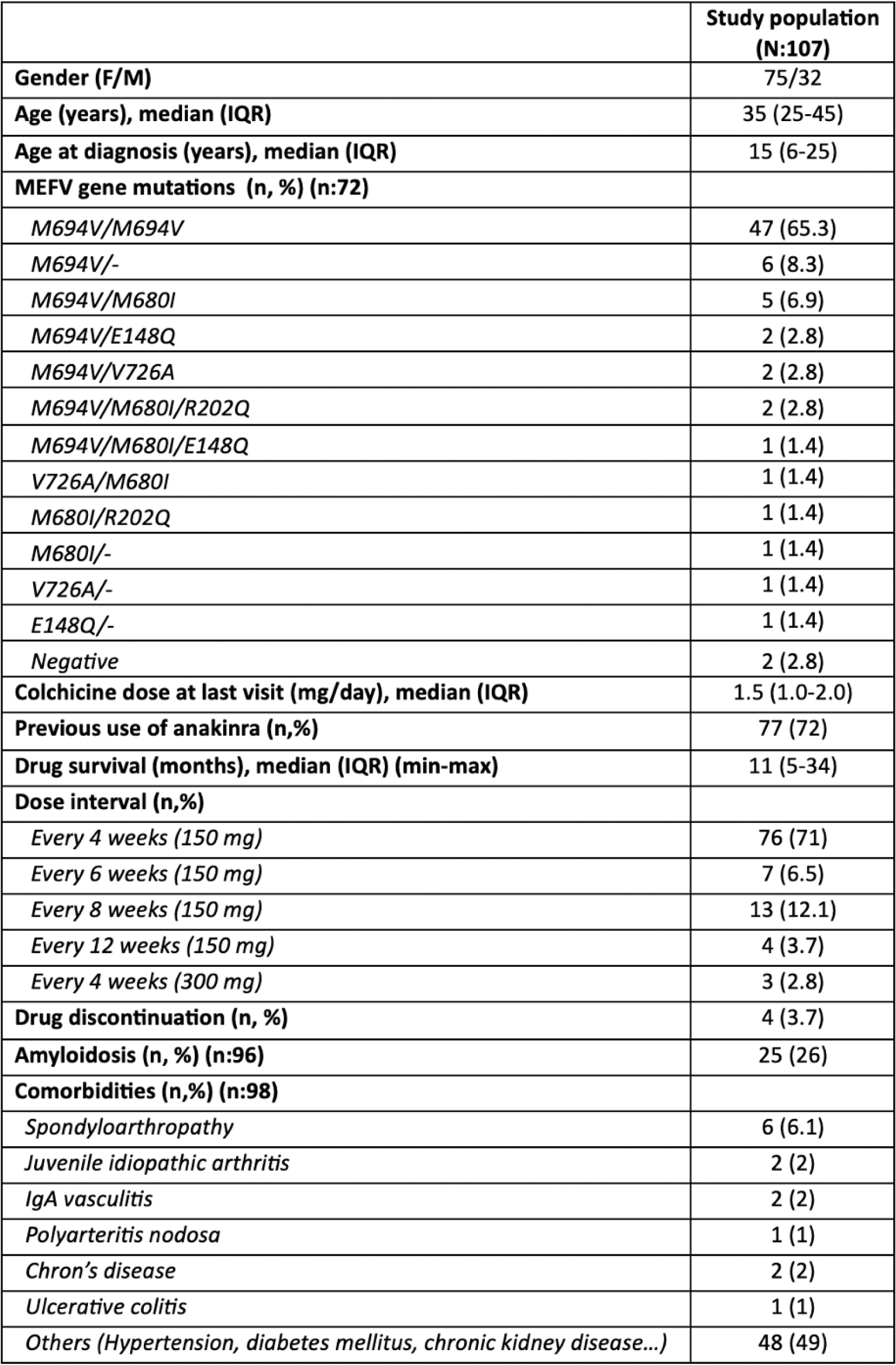
Results: A total of 107 patients with FMF (75 F, 32 M) were followed with canakinumab therapy. The median age at diagnosis was 15 (6-25) years, while the median age of the cohort was 35 (25-45) years. The most common MEFV gene mutation identified was M694V/M694V. Twenty-five patients were identified to have amyloidosis, and 62 patients had associated comorbidities. The maximum duration of canakinumab use was 128 months. In 27 (25.2%) patients, dosing intervals were successfully extended beyond the standard 4-week schedule, while in three patients, the dose was increased to 300 mg/month. Abdominal pain, headache, back pain, upper respiratory tract infection, skin rash, fever, and artralgia were the common adverse events. Notably, no patient discontinued the drug due to adverse effects. Four patients discontinued canakinumab treatment due to the following reasons: a planned pregnancy in one patient, initiation of an alternative biologic DMARD for Crohn’s disease in another, lack of efficacy in one patient, and changes in insurance coverage conditions in the remaining case.
Conclusion: In real-world clinical practice, canakinumab treatment has demonstrated favourable efficacy and safety profiles, with a high retention rate. The treatment was well tolerated, even in patients with comorbidities, and there were no discontinuations due to adverse effects. Dose adjustment has worked in one-quarter of the patients. Further studies are needed to obtain safety data in specific populations, such as during pregnancy.
Table 1. Demographic and clinical characteristics of study population
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (