

Background: VEXAS syndrome is caused by somatic UBA1 -mutations in the X-chromosome in hematopoietic cells, characterized by dysplasia and inflammatory symptoms [1]. Limited previous data have shown promising results with azacitidine treatment [2].
Objectives: The aim of this study is to evaluate the effectiveness of azacitidine treatment defined in complete and partial response (CR; PR) and the reduction of the lymphocyte corrected variant allele frequency (cVAF) of the UBA1 -mutation in a cohort of patients with VEXAS syndrome.
Methods: Clinical and laboratory data from 21 VEXAS patients were retrospectively evaluated, thirteen were treated with azacitidine (75 mg/m 2 QD for seven days every 28 days). Because mature lymphocytes do not contain the UBA1 -mutation, a corrected VAF (cVAF) was calculated.
Results: Of the patients not treated with azacitidine (N=8) no follow-up data is available, due of death, treatment elsewhere or alternative treatment. Ten patients treated with azacitidine showed clear cVAF decreases, all patients had clinical benefit. Four patients achieved CR (cVAFs <1%) with >50% CRP decrease, and no or very low prednisolone dose. Ultimately azacitidine could be stopped successfully. Six patients showed PR, cVAFs decreases ranging from 55-96%, prednisolone could be tapered to 0-7.5 mg QD and four of them showed >50% CRP decrease. Two patients showed little cVAF decreases of 7 and 10%, needing medium doses of prednisolone (10-15 mg QD) with one of them showing >50% CRP decrease.
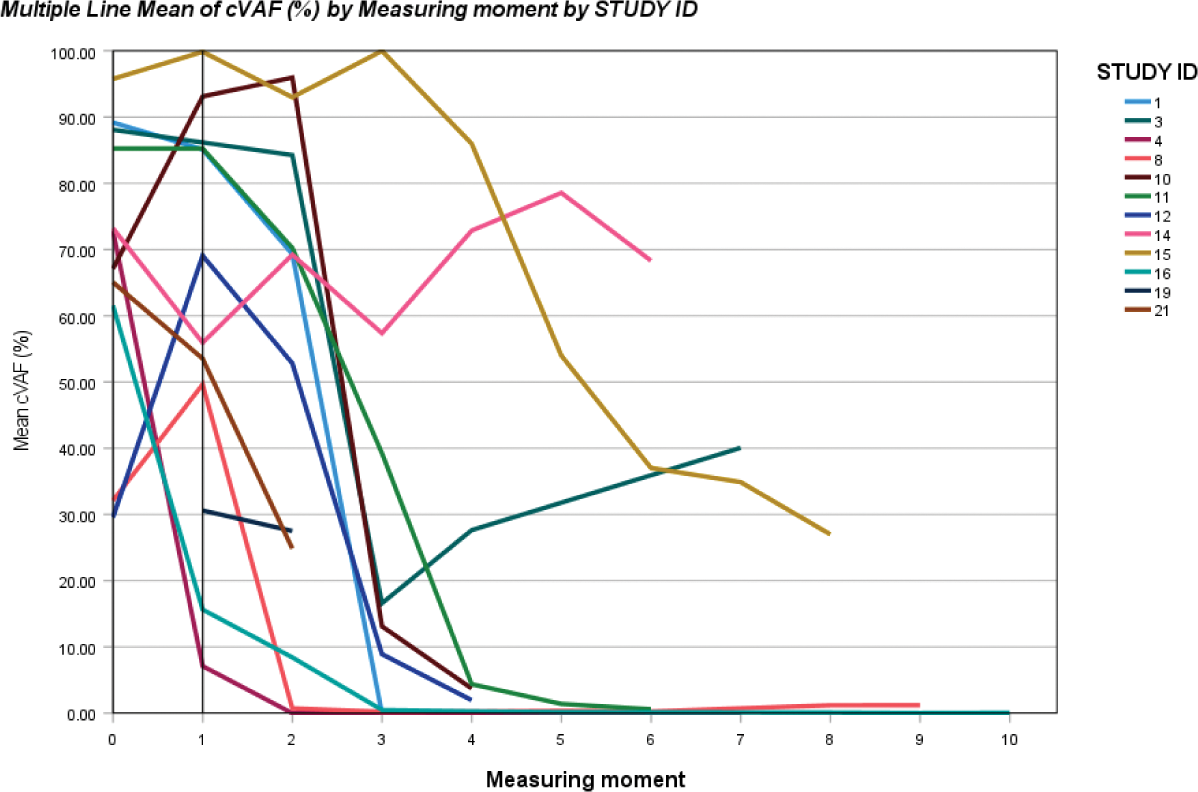
Conclusion: Azacitidine treatment reduces the cVAF of the UBA1 -mutation, and induces recovery of inflammatory symptoms in most VEXAS patients. If CR is achieved azacitidine can be stopped successfully.
REFERENCES: [1] Beck DB, et al. Somatic Mutations in UBA1 and Severe Adult-Onset Autoinflammatory Disease. N Engl J Med. 2020 Dec 31;383(27):2628-2638.
[2] Aalbers AM, et al. Long-term genetic and clinical remissions after cessation of azacitidine treatment in patients with VEXAS syndrome. Hemasphere. 2024 Jul 30;8(8):e129.
Acknowledgements: NIL.
Disclosure of Interests: Sanne Wijnberger: None declared, Myrthe Natzijl: None declared, Julie Weering: None declared, Jasmijn Meerlo: None declared, Jonas Salzbrunn: None declared, Andre Mulder: None declared, Saskia Klein: None declared, Abraham Rutgers Not related to the data presented.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (