

Background: Complement consumption, characterized by decreased C3 and/or C4 levels, is a hallmark of immune complex-mediated inflammation and vascular involvement in patients with systemic autoimmune diseases. In patients with Sjögren Disease (SjD), hypocomplementemia at diagnosis has been mainly linked to an increased risk of lymphoma. By analysing the relationship between complement consumption profiles and systemic activity in the largest international cohort, we aim to refine early prognostic paradigms and inform tailored clinical surveillance strategies in SjD, thereby addressing a critical gap in precision medicine for this complex autoimmune disease.
Objectives: The objectives of this study were to identify and classify complement consumption patterns, investigate their association with an early phenotype consisting of systemic activity across ESSDAI domains while adjusting for age and gender, and explore how cross-validation techniques may validate the predictive accuracy and generalizability of developed models.
Methods: This study analyzed data from the International Sjögren Big Data Registry. Patients were categorized into four distinct groups according to their complement consumption patterns (isolated low C3, isolated low C4, combined low C3 and C4, and normal C3 and C4 levels). We used Chi-square tests to evaluate univariate associations, and Kruskal-Wallis H-test and Mann-Whitney U test to investigate significant differences with respect to systemic activity (mean ESSDAI score and DAS categories). Multivariable logistic regression models were developed to analyze associations between complement patterns and ESSDAI domains, adjusting for age and gender. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. A five-fold stratified cross-validation was carried out to rigorously evaluate the models’ generalizability, with the Area Under the Curve (AUC) serving as the primary performance metric. Generative Artificial Intelligence (AI) (ChatGPT-4o model) was used within a secure offline environment to automate anonymized data recoding and statistical scripting. Python libraries, including pandas, statsmodels, and scikit-learn, were integral for data processing, model development, and cross-validation.
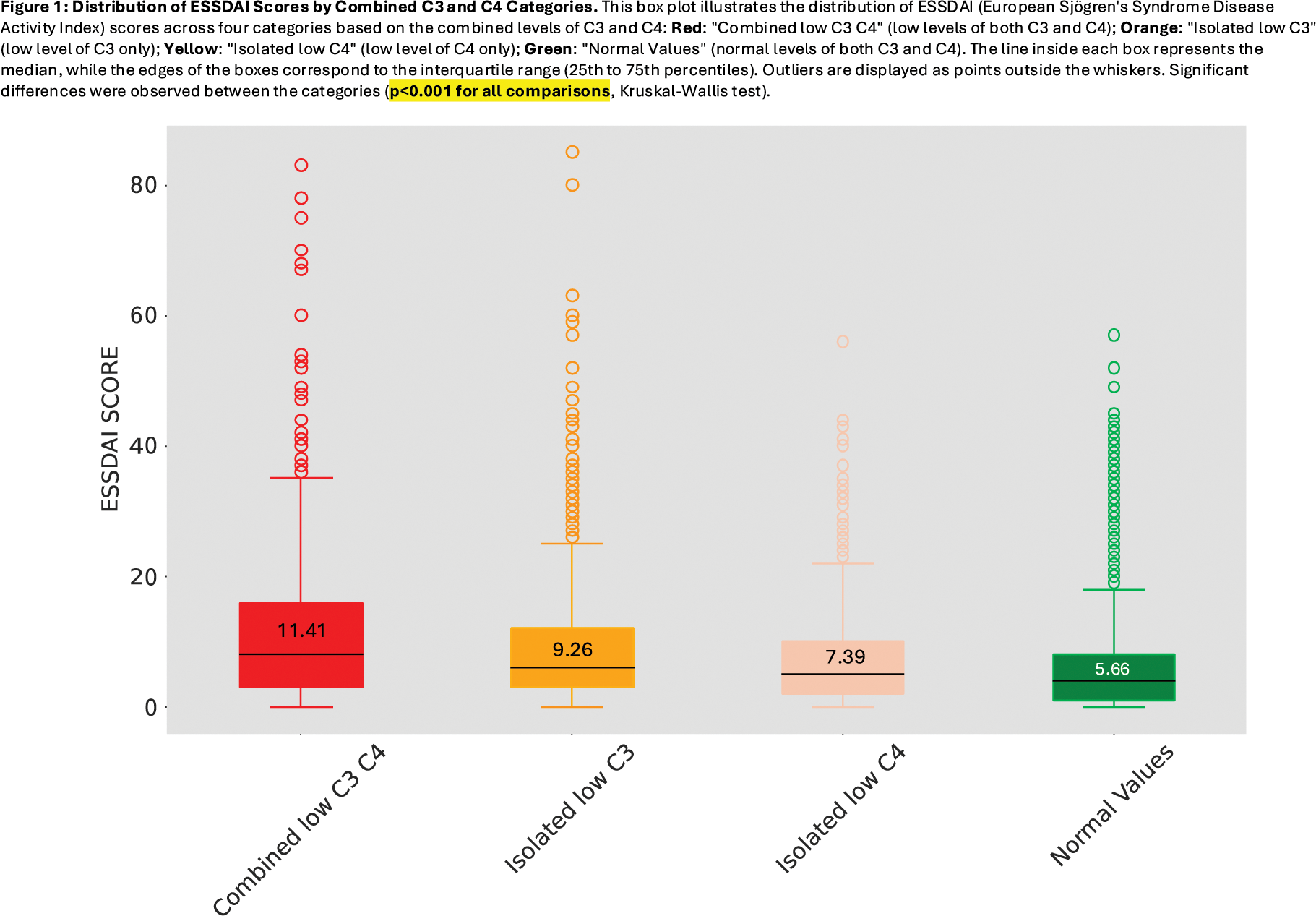
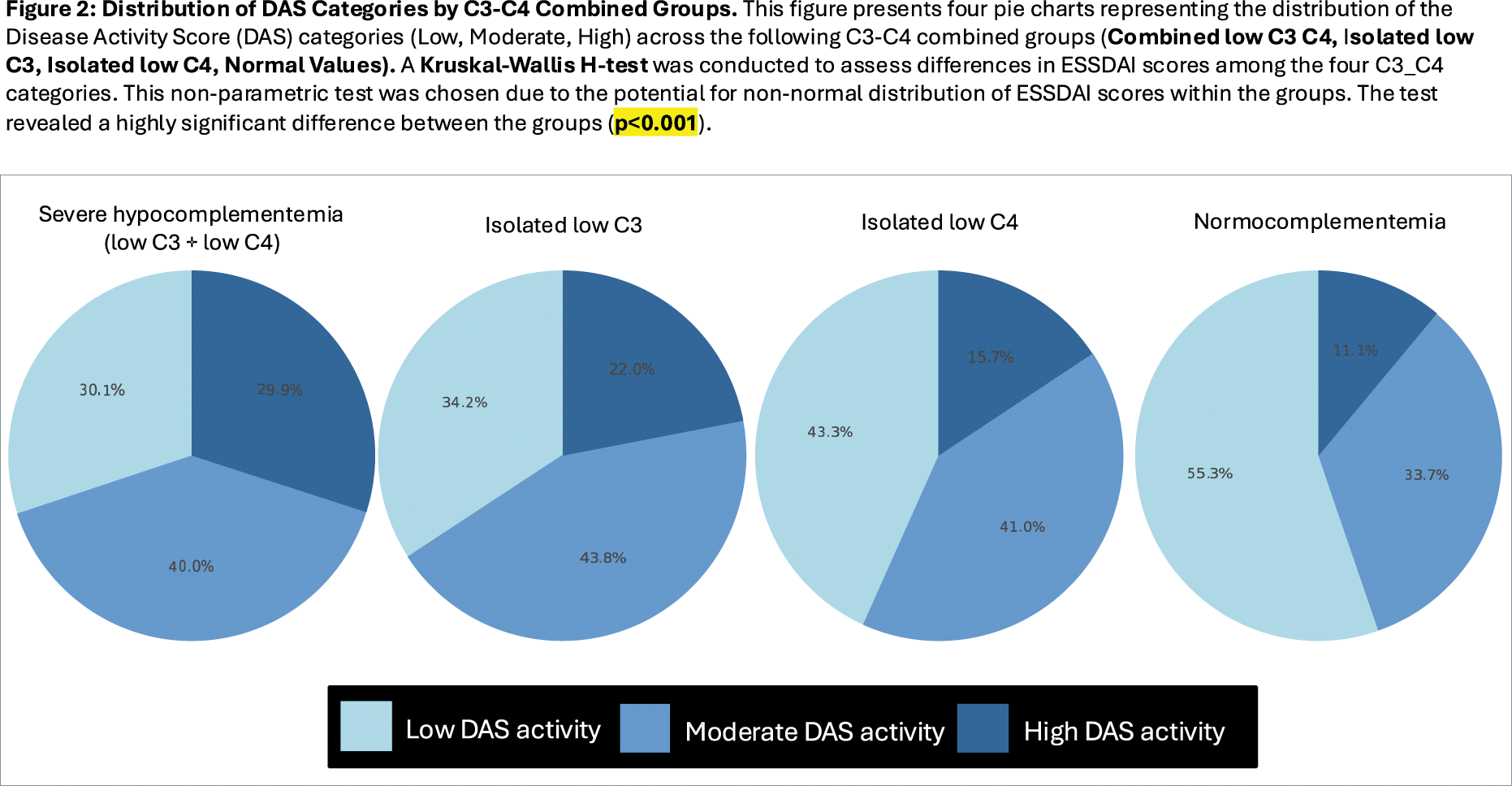
Results: Complement values determined at diagnosis were available in 13,710 patients. Stratification according to the 4 complement consumption patterns identified that 79.58% of patients had normal levels of C3 and C4, 7.42% exhibited isolated low C3, 6.90% showed isolated low C4, and 6.09% presented combined low C3 and C4 levels. Combined low C3-C4 levels exhibited the highest mean ESSDAI score (11.41), follwed by isolated low C3, isolated low C4 and normocomplementemia (mean ESSDAI score of 9.26, 7.39, and 5.66, respectively) (Figure 1); Kruskal-Wallis H-test revealed a highly significant difference between the groups (p<0.001), as well as pairwise comparisons using the Mann-Whitney U test (p<0.001). The Chi-square test revealed significant differences in the distribution of DAS categories across the C3-C4 combined groups (χ2=476.41, p<0.001) (Figure 2). However, multivariate logistic regression confirmed significant associations in only three domains. For the pulmonary domain, combined low C3 and C4 levels were associated with the highest odds of activity (OR: 3.12, 95% CI: 2.50–3.91, p < 0.001; AUC: 0.591). In the biological domain, isolated low C3 strongly correlated with activity (OR: 2.45, 95% CI: 1.98–3.03, p < 0.001; AUC: 0.580). For constitutional symptoms, isolated low C3 was associated with the highest activity frequency (18.54%), whereas normal complement levels showed the lowest frequency (11.06%, OR: 0.56, 95% CI: 0.48–0.67, p < 0.001; AUC: 0.563). After adjusting for epidemiological factors, sex and age emerged as influential variables: men had higher odds of constitutional activity (OR 1.24, 95% CI: 1.02–1.52, p = 0.03), while older age had a protective effect, reducing systemic activity by about 1% per year (OR: 0.99, 95% CI: 0.99–1.00, p = 0.0003). The AUC values obtained after running the five-fold stratified cross-validation generalizability models ranged between 0.56 and 0.59, indicating modest ability to discriminate between active and inactive states.
Conclusion: This study demonstrates that complement consumption patterns are strongly associated with baseline systemic activity in SjD, highlighting their potential as early prognostic markers. While complement patterns provide valuable insights for risk stratification, the current predictive models exhibit modest discriminatory ability (AUC values between 0.5 and 0.6), suggesting that complement patterns are relevant but insufficient alone as predictors to improve clinical applicability. The nuanced influence of epidemiological factors—such as the protective effect of age and the increased susceptibility of men to systemic disease—adds complexity to our understanding of early systemic Sjögren.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (