

Background: Different causes such as inflammatory processes, pain and disease activity might be attributed to less physical activity and a higher body fat percentage [2], resulting to limitations in physical performance [1] in patients with inflammatory rheumatic musculoskeletal diseases.
Objectives: To study obesity and abnormal phenotypes of body composition in patients with axial SpA (axSpA) or psoriatic arthritis (PsA) and assess the associations between obesity and physical performance.
Methods: Consecutive patients with axSpA and PsA either on conventional treatment or Tumor-necrosis factor inhibitors (TNFi) underwent a standardized assessment on patient and disease characteristics and a battery of performance-based tests including short physical performance battery (SPPB), the ankylosing spondylitis performance index (ASPI), grip strength and gait speed. Body composition was assessed using dual x-ray absorptiometry (DXA), where appendicular lean mass (ALM) index (ALMI) and body fat percentage were determined. Obesity was defined as a body fat percentage ≥30% (male) or ≥40% (female) patients and sarcopenia as ALM/BMI <0.789 (male) or <0.512 (female) patients. Limited physical performance was classified by an SPPB score ≤8, gait speed ≤0.8m/s and grip strength <16kg (female) and <27kg (male). Uni- and multivariable linear regression analyses were used to examine the associations between body fat percentage (dependent variable) and various patients’ characteristics (independent variables).
Results: A total of 205 patients (132 axSpA (64.4%), 73 PsA (35.6%)) were included and 127 patients (59.6%) were treated with TNFi (Table 1). 116 (56.6%) were classified as obese (52.3% axSpA, 64.4% PsA). Obese patients had higher disease activity, impaired physical function but reported similar levels of physical activity compared to non-obese patients (Table 1). Elevated body fat percentage was numerically higher in PsA and was significantly less frequent in patients on TNFi. Physical performance was impaired in obese patients compared to non-obese patients (Table 1). 15 (12.9%) of the obese patients couldn’t complete one or more ASPI tests, while 3 (3.4%) of non-obese subjects failed to do so. 18 (15.5%) obese patients scored ≤8 points in the SPPB, compared to 6 (6.7%) of non-obese patients. Gait speed was reduced in 12 (10.3%) and grip strength in 14 (12.1%) of obese patients and gait speed not at all in non-obese subjects, while grip strength was reduced in 7 (7.9%) patients with a worse ASPI result, are significantly more often obese, while this connection was only found for male patients in the SPPB (Table 1). ALM/BMI was significantly lower in the obese group, and more obese patients were sarcopenic. Obese subjects had a significantly higher global patient assessment, pain level, ASDAS, and lower BASFI. CRP levels were significantly elevated in male obese patients, while those being treated with TNFi were were less likely to be obese. In females, obese subjects had significantly lower gait speed and showed pathological findings more often. Predictors of a higher body fat percentage in male patients included longer symptom duration and conventional treatment (Table 2). In female patients the predictors were higher age, and more time needed to complete the ASPI (Table 2).
Conclusion: The prevalence of obesity was high among patients with SpA and was associated with impaired physical performance. Key predictors for higher body fat percentage included longer symptom duration, conventional treatment, older age, and worse performance measured by the ASPI. Male patients treated with TNFi exhibited a lower body fat percentage while longer disease duration was correlated with higher body fat percentage. Impaired physical performance, especially in the ASPI, and higher age were significant contributors to these effects in female patients.
REFERENCES: [1] Khoja SS. Arthritis Care Res (Hoboken). 2018 Mar;70(3):333-342.
[2] Giles JT Arthritis Rheum. 2008;59(6):807-815.
Table 1. Comparison between obese and non-obese SpA patients
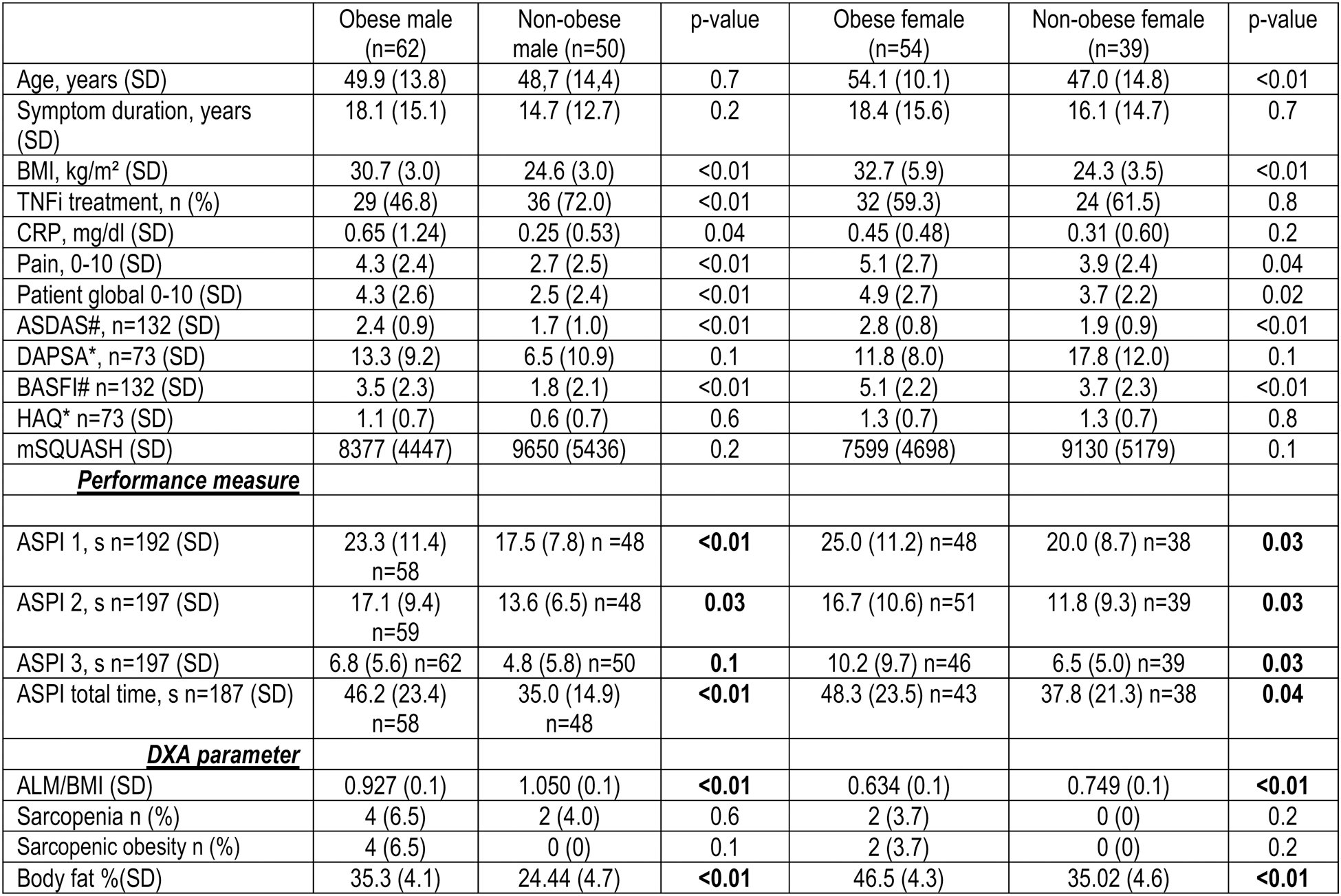
#axSpA patients; *PsA patients.
Table 2. Association of body fat percentage and various patient characteristics.
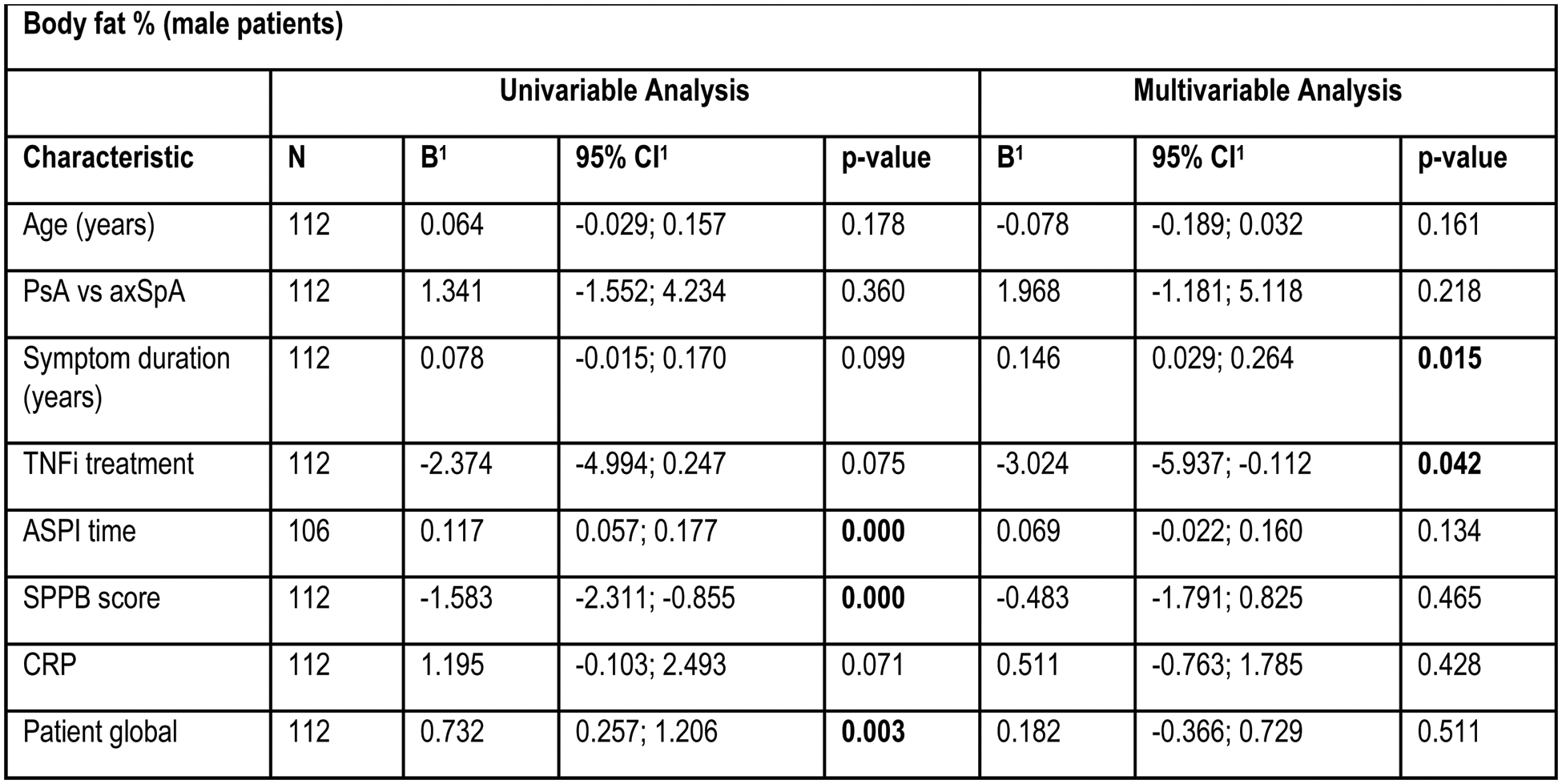
B=regression coefficient; CI = Confidence Interval.
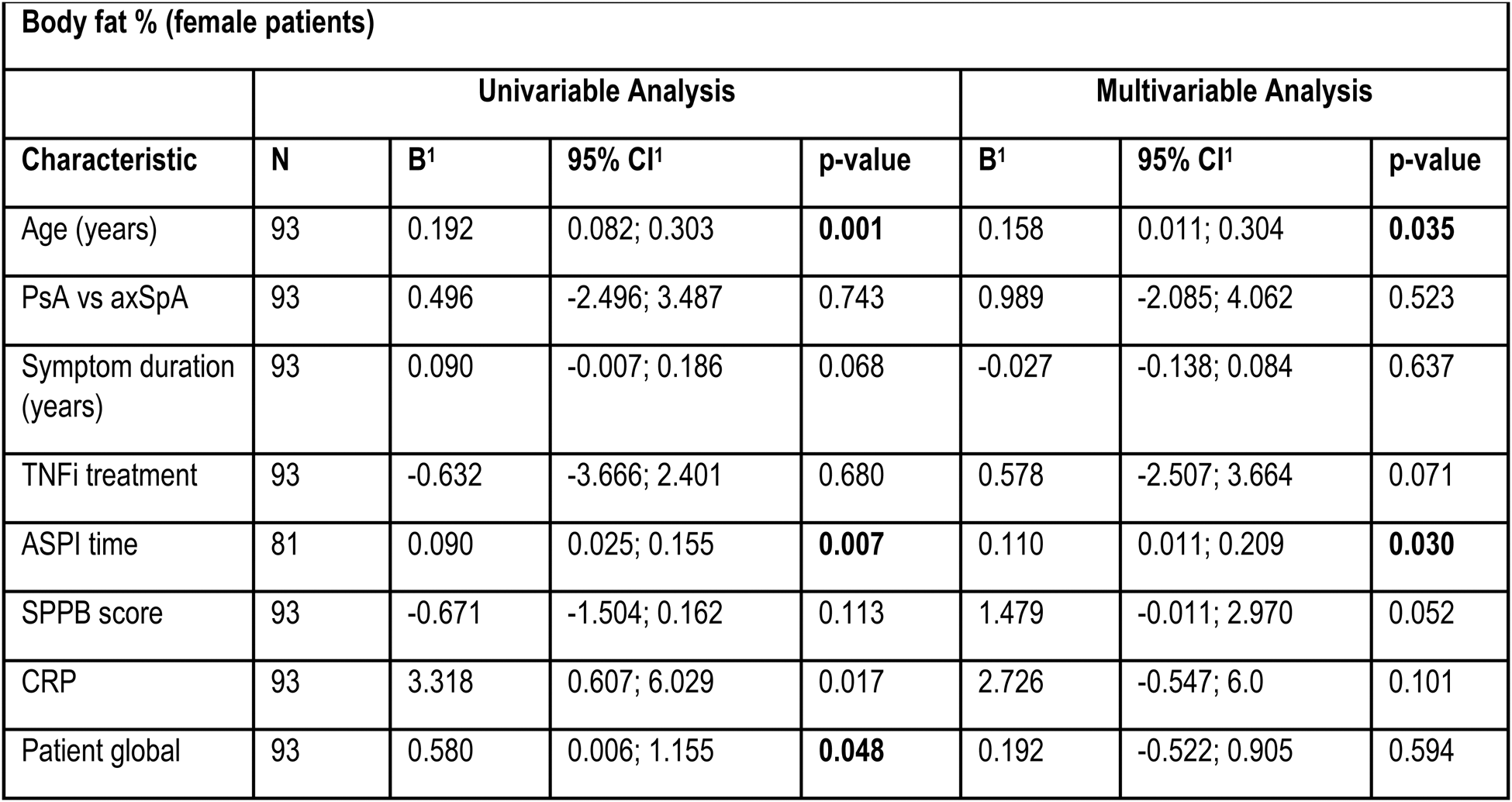
B=regression coefficient; CI = Confidence Interval.
Acknowledgements: The study was funded by Fresenius Germany.
Disclosure of Interests: Uta Kiltz AbbVie, Amgen, Eli Lilly, Fresenius, GSK, Hexal, Janssen, MSD, Novartis, Pfizer, UCB, AbbVie, Eli Lilly, Fresenius, GSK, Hexal, Janssen, MSD, Novartis, UCB, Amgen, Fresenius, GSK, Hexal, Novartis, Marius Bedei: None declared, Iuliia Kononenko: None declared, Imke Redeker: None declared, David Kiefer AbbVie, Eli Lilly, Galapagos, Janssen, Novartis and UCB, AbbVie, Eli Lilly, Galapagos, Janssen and UCB, Novartis, Philipp Sewerin AXIOM Health, AMGEN, AbbVie, Biogen, Bristol-Myers Squibb, Celgene, Chugai Pharma Marketing Ltd./ Chugai Europe, Deutscher Psoriasis-Bund, Gilead Sciences, Hexal Pharma, Janssen-Cilag, Johnson & Johnson, Lilly/ Lilly Europe/ Lilly Global, medi-login, Mediri GmbH, Novartis Pharma, Onkowissen GmbH, Pfizer, Roche Pharma, Rheumazentrum Rhein-Ruhr, Sanofi-Genzyme, Spirit Medical Communication, Swedish Orphan Biovitrum, UCB Pharma, AXIOM Health, AMGEN, AbbVie, Biogen, Bristol-Myers Squibb, Celgene, Chugai Pharma Marketing Ltd./ Chugai Europe, Deutscher Psoriasis-Bund, Gilead Sciences, Hexal Pharma, Janssen-Cilag, Johnson & Johnson, Lilly/ Lilly Europe/ Lilly Global, medi-login, Mediri GmbH, Novartis Pharma, Onkowissen GmbH, Pfizer, Roche Pharma, Rheumazentrum Rhein-Ruhr, Sanofi-Genzyme, Spirit Medical Communication, Swedish Orphan Biovitrum, UCB Pharma, AXIOM Health, AMGEN, AbbVie, Biogen, Bristol-Myers Squibb, Celgene, Chugai Pharma Marketing Ltd./ Chugai Europe, Deutscher Psoriasis-Bund, Gilead Sciences, Hexal Pharma, Janssen-Cilag, Johnson & Johnson, Lilly/ Lilly Europe/ Lilly Global, medi-login, Mediri GmbH, Novartis Pharma, Onkowissen GmbH, Pfizer, Roche Pharma, Rheumazentrum Rhein-Ruhr, Sanofi-Genzyme, Spirit Medical Communication, Swedish Orphan Biovitrum, UCB Pharma, Ioana Andreica UCB, Astra Zeneca, Chugai, Amgen, Abbvie, Alfa Sigma, Astra Zeneca, Chugai, Gilead, GSK, Janssen, Lilly, MSD, Novartis, Pfizer, Sobi, UCB, Amgen, Abbvie, Alfa Sigma, Astra Zeneca, Boehringer Ingelheim, Chugai Pharma, Galapagos, Lilly, Novartis, Sobi, UCB, Takeda, Lilly, Anna L. Kernder GSK, Vifor, Astra Zeneca, Astra Zeneca, unrestricted grants from UCB Pharma and GSK, Diana Vossen Bristol-Myer Squibb, Celgene GmbH, Chugai Pharma GmbH,Gilead Sciences Inc., GlaxoSmithKline plc, Lilly Deutschland GmbH, Medac GmbH, Novartis Pharma GmbH, Pfizer Deutschland GmbH, Abbvie, UCB, Amgen, Boehringer Ingelheim Pharma GmbH & Co. KG, Björn Bühring Amgen, AstraZeneca, Biogen, Boehringer–Ingelheim, Gilead/Galapagos, Merck Sharpe. Dohme, Sanofi Genzyme, Theramex and UCB, AbbVie, Alexion, Amgen, AstraZeneca, Fresenius-Kabi, Gilead/Galapagos, Janssen, Theramex and UCB, Xenofon Baraliakos Abbvie, Alphasigma, Amgen, BMS, Cesas, Celltrion, Galapagos, Janssen, Lilly, Moonlake, Novartis, Pfizer, Roche, Sandoz, Springer, Stada, Takeda, UCB, Zuellig, Abbvie, Alphasigma, Amgen, BMS, Cesas, Celltrion, Galapagos, Janssen, Lilly, Moonlake, Novartis, Pfizer, Roche, Sandoz, Springer, Stada, Takeda, UCB, Zuellig, Abbvie, Alphasigma, Amgen, BMS, Cesas, Celltrion, Galapagos, Janssen, Lilly, Moonlake, Novartis, Pfizer, Roche, Sandoz, Springer, Stada, Takeda, UCB, Zuellig.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (