

Background: Interstitial lung disease associated with Sjögren’s disease (SjD-ILD) represents a major extra-glandular manifestation for patients with SjD and is linked to decreased survival rates. The absence of evidence from randomized clinical trials and comparative observational studies on the use of immunosuppressive therapy for SjD-ILD patients hampers the recommendation of specific treatment options in this context. Furthermore, there is a lack of information on the current treatment practices for patients with SjD-ILD in real-world settings.
Objectives: To assess treatment patterns in SjD-ILD across a multicenter European cohort.
Methods: A post-hoc analysis of prospectively collected data of SjD-ILD patients from three European expert centers (Oslo University Hospital, Medical University of Vienna, and the University Hospital Zurich) with available high resolution computed tomography (HRCT) was conducted. ILD diagnosis and pattern assessment on HRCT was conducted by expert radiologists. Data collected included demographics, extrapulmonary manifestations, pulmonary function test results (FVC, DLCO), ILD pattern (e.g., nonspecific interstitial pneumonia [NSIP], lymphocytic interstitial pneumonia [LIP], and others), and treatment regimens. Treatment patterns were categorized into monotherapy (a single disease-modifying antirheumatic drug [DMARD]) and combination therapy (two or more DMARDs). Descriptive statistics were used to summarize treatment patterns and associated clinical data.
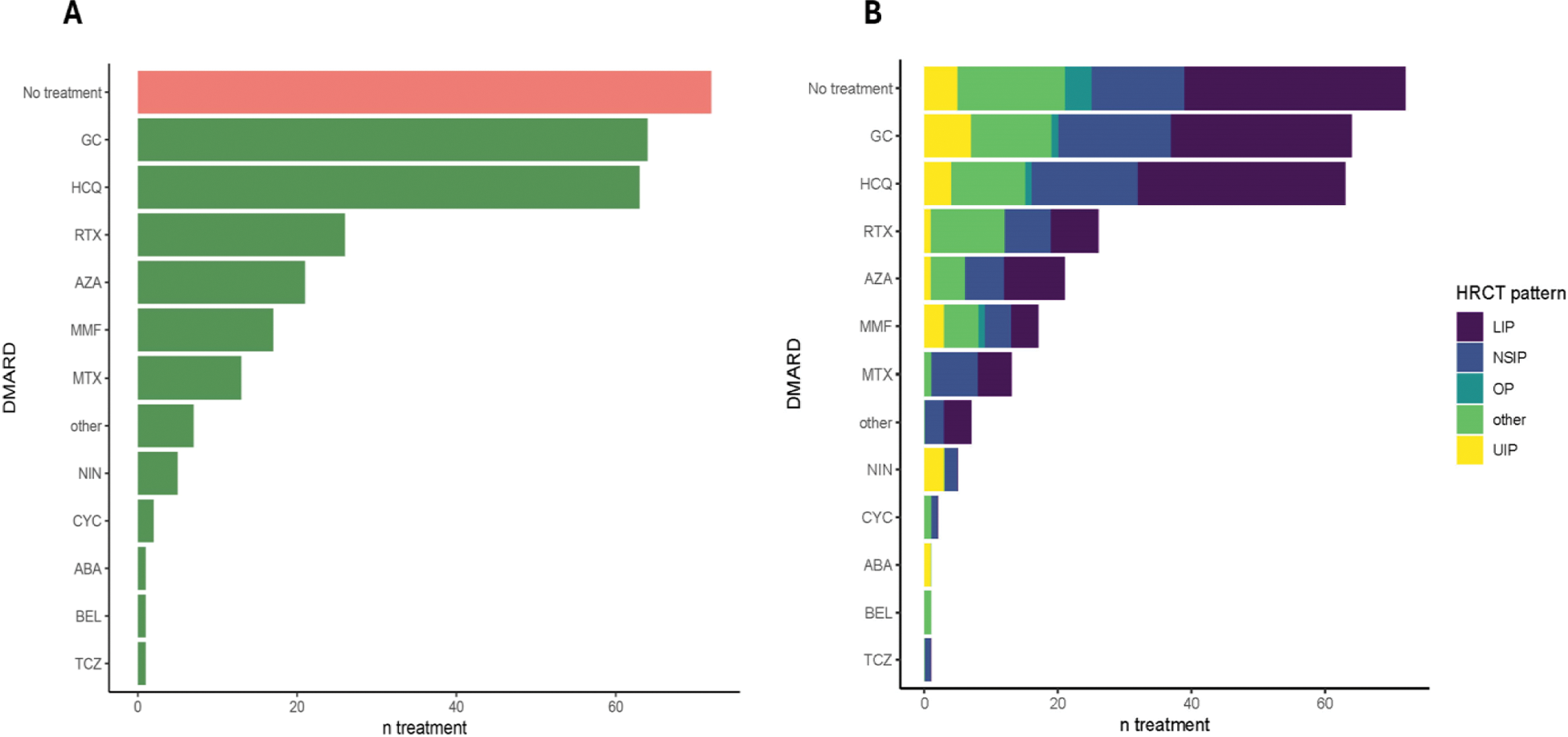
Results: Among 547 patients with SjD and available HRCT, 163 (29.8%) had confirmed ILD. The cohort was predominantly female (82.8%), with a mean age of 68.0 ± 13.0 and mean disease duration of 6.2 ± 5.6 years. LIP (44.8%) and NSIP (22.7%) were the most frequent ILD subtypes, while UIP accounted for 9.2% (Table 1). Mean FVC was 92.9 ± 20.3%, and DLCO was 71.0 ± 20.2%. Glucocorticoids (GC) (39.3%) were the most frequently prescribed treatment, followed by rituximab (RTX) (16.0%), azathioprine (AZA) (12.9%), and mycophenolate mofetil (MMF) (10.4%) (Figure 1A). Monotherapy with a single DMARD was seen in 16.0%, while 9.2% received combination therapy, primarily MMF or methotrexate with RTX. When assessing the used therapies segregated by ILD pattern, Nintedanib use was observed in UIP patients (20%), while rituximab was more frequent in cases classified as other patterns, such as bronchiolitis (44%) (Figure 1B).
Conclusion: This study highlights the variability in treatment patterns for SjD-ILD, emphasizing the importance of studies assessing treatment efficacy in SjD-ILD. Our findings underscore the need for standardized treatment protocols and further research to refine and optimize management strategies for SjD-ILD.#.
REFERENCES: [1] Flament T, Bigot A, Chaigne B, Henique H, Diot E, Marchand-Adam S. Pulmonary manifestations of Sjögren’s syndrome. Eur Respir Rev. 2016;25(140):110-23.
Baseline characteristics of the Sjögren’s disease-associated ILD (SjD-ILD)-multicenter cohort. The table includes demographic data (age, sex), disease duration, treatment, and pulmonary function metrics (FVC, DLCO).
| SjD-ILD patients | ||
|---|---|---|
| No. (n) | 163 | |
| Sex (female) | 135 (82.8%) | |
| Mean age (years ± SD) | 68.0 ± 13.0 | |
| Ethnicity | ||
| Caucasian | 154 (94.5%) | |
| Asian | 6 (3.7%) | |
| African | 3 (1.8%) | |
| Mean Disease duration (years ± SD) | 6.2 ± 5.6 | |
| CRP in mg/dl | 0.6 ± 1.0 | |
| ESR in mm/h | 34.2 ± 24.2 | |
| Autoantibodies | ||
| SSA/Ro | 138 (84.7%) | |
| SSB/La | 96 (58.9%) | |
| Seronegative | 9 (5.5%) | |
| Extraglandulary manifestations | ||
| Arthritis | 17 (10.4%) | |
| Raynaud‘s phenomenon | 33 (20.2%) | |
| Lymphadenopathy | 37 (22.7%) | |
| Lymphopenia | 56 (34.4%) | |
| CNS affection | 3 (1.8%) | |
| Vasculitis | 20 (12.3%) | |
| Fatigue | 63 (38.7%) | |
| Dermal affection | 8 (4.9%) | |
| Interstitial nephritis | 8 (4.9%) | |
| Pulmonary function tests (mean ± SD) | ||
| FVC % | 92.9 ± 20.3 | |
| DLCO % | 71.0 ± 20.2 | |
| NYHA stage (median, IQR) | 2.0 (1.0, 2.0) | |
| ILD-Subtype | ||
| NSIP | 37 (22.7%) | |
| LIP | 73 (44.8%) | |
| UIP | 15 (9.2%) | |
| OP | 5 (3.1%) | |
| Other/ missing | 25 (15.3%) | |
| Immunosuppressive/antifibrotic therapy | ||
| 1 DMARD | 26 (16.0%) | |
| 2 DMARDs | 15 (9.2%) | |
| MMF+RTX | 4 | |
| MTX+RTX | 4 | |
| AZA+RTX | 2 | |
| MMF+CSP | 1 | |
| MMF+TAC | 1 | |
| MMF+NIN | 1 | |
| MMF+AZA | 1 | |
| TCZ+NIN | 1 | |
| 3 DMARDs | 1 (0.6%) | |
| MMF+ABA+ NIN | 1 | |
Treatment Patterns in Patients with Sjögren’s Disease-Associated ILD (SjD-ILD ). (A ) Overview of therapies used in the SjD-ILD cohort, including corticosteroids (GC), hydroxychloroquine (HCQ), rituximab (RTX), azathioprine (AZA), mycophenolate mofetil (MMF), methotrexate (MTX), nintedanib (NIN), cyclophosphamide (CYC), abatacept (ABA), belimumab (BEL), and tocilizumab (TCZ). The bar lengths represent the number of patients receiving each treatment. (B ) Treatment patterns stratified by high-resolution computed tomography (HRCT) subtypes, including lymphocytic interstitial pneumonia (LIP), nonspecific interstitial pneumonia (NSIP), organizing pneumonia (OP), usual interstitial pneumonia (UIP), and other patterns (such as bronchiolitis).
Acknowledgements: NIL.
Disclosure of Interests: Kastriot Kastrati: None declared, Marco Sprecher Grant/research support from: AbbVie, Emily Langballe Speakers fee – Boehringer-Ingelheim, Phuong Phuong Diep ndependent grants and lecture fees from Boehringer-Ingelheim, Attended advisory board meeting with NordicInfu Care AB, Håvard Fretheim Speakers Bureau: Boehringer Ingelheim, Helena Andersson: None declared, Paul Studenic AbbVie, Daniel Aletaha: None declared, Lena-Theresa Göthans: None declared, Karolina Anderle: None declared, Bojana Müller: None declared, Cathrine Brunborg: None declared, Dr. Cosimo Bruni Educational grants from Wellcome Trust. Congress support from Boehringer-Ingelheim, Hartmann-Muller Foundation, Boehringer Ingelheim, Novartis Foundation for medical-biological research, EMDO Foundation, Iten-Kohaut Foundation, Christian Clarenbach Boehringer Ingelheim, Roche, Thomas Frauenfelder Bayer, Trond Mogens Aaløkken: None declared, Natasha Moe Boehringer Ingelheim, Helmut Prosch AstraZeneca, BMS, Boehringer Ingelheim, Bracco, Daiichi Sankyo, Janssen, MSD, Novartis, Roche, Sanofi, Siemens Healthineers, Takeda. Travel grants: Boehringer Ingelheim, BMS, Boehringer Ingelheim, Janssen, MSD, Roche, Sanofi, Boehringer Ingelheim, AstraZeneca, Siemens Healthineers and the Christian Doppler Research Association, EU Commission (EU4Health, Horizon Europe Health), Øyvind Molberg: None declared, Oliver Distler 4P-Pharma, Abbvie, Acceleron, Acepodia Biotech, Aera, Alcimed, Altavant, Amgen, AnaMar, Anaveon AG, Argenx, AstraZeneca, Blade, Bayer, Boehringer Ingelheim, Calluna (Arxx), Cantargia AB, Catalyze Capital, Corbus, CSL Behring, Galderma, Galapagos, Glenmark, Gossamer, Horizon, Janssen, Kymera, Lupin, Medscape, MSD Merck, Miltenyi Biotec, Mitsubishi Tanabe, Nkarta Inc., Novartis, Orion, Pilan, Prometheus, Quell, Redxpharma, Roivant, EMD Serono, Topadur and UCB. Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143). Co-founder of CITUS AG., BI, Kymera, Mitsubishi Tanabe, UCB, Helga Lechner-Radner Abbvie, Gilead, Janssen, Merck-Sharp, Pfizer, UCB-Pharma, Anna-Maria Hoffmann-Vold Boehringer Ingelheim, Janssen, Medscape, Merck Sharp & Dohme, Novartis and Roche, Abbvie, ARXX, Boehringer Ingelheim, Bristol Myers Squibb, Genentech, Janssen, Medscape, Merck Sharp & Dohme, Pliant therapeutics, Roche and Werfen, Boehringer Ingelheim, Janssen.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (