

Background: Nintedanib is an antifibrotic drug that has proven effective in the treatment of idiopathic pulmonary fibrosis (IPF) and pulmonary fibrosis associated with immune-mediated diseases such as systemic sclerosis and rheumatoid arthritis. Its use has transformed the management of these manifestations, offering patients a therapeutic option that may slow disease progression.
Objectives: To evaluate the tolerance of Nintedanib in patients with diffuse interstitial lung disease (DILD) associated with immunomediated diseases (IMD-DILD) and compare it with the tolerance in patients with DILD associated with non-immunomediated diseases (non-IMD-DILD).
Methods: Descriptive observational study that includes data from patients diagnosed with DILD under follow-up in a multidisciplinary Pneumology and Rheumatology practice integrated into a DILD unit between May 2019 and September 2024. Demographic data, adverse effects of Nintedanib and causes of withdrawal were collected and compared between both groups. Quantitative variables were described as mean and standard deviation, and frequencies and percentages were used for qualitative variables. Comparison of proportions was performed using the chi-square test. P-values <0.05 were considered statistically significant.
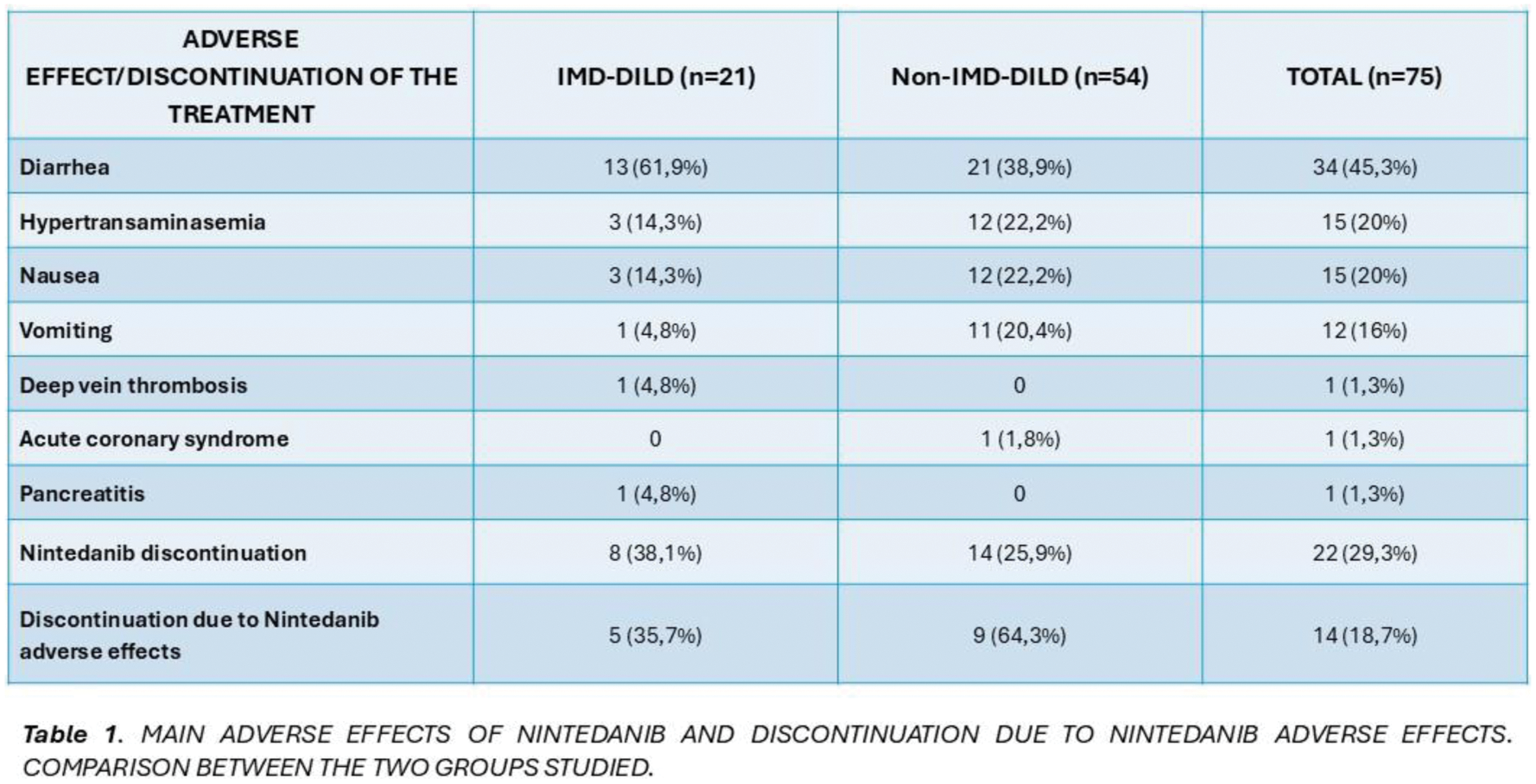
Results: A total of 75 patients were included, 54.7% male and 45.3% female. The mean age was 70.9 ± 11.65 years. 54 (72%) had non-IMD-DILD and 21 (28%) IMD-DILD. In the IMD-DILD group, 9 were diagnosed with systemic sclerosis, 3 with rheumatoid arthritis, 3 with overlap syndrome, 2 with antisynthetase syndrome, 1 with ANCA MPO vasculitis, 1 with dermatomyositis, 1 with IPAF (interstitial pneumonia with autoimmune features) and 1 with Sjögren’s syndrome. Of the total number of patients, 54 (72%) presented adverse effects; 34 (45.3%) diarrhea, 15 (20%) hypertransaminasemia, 15 (20%) nausea and 12 (16%) vomiting (Table 1). One patient presented pancreatitis, another deep vein thrombosis, and another presented acute coronary syndrome. The occurrence of diarrhea was higher in patients with IMD-DILD (61.9% vs. 38.9%) without reaching statistical significance (p=0.072). The occurrence of hypertransaminasemia was greater in the non-IMD-DILD group (22.2% vs 14.3%), as well as the presence of nausea (22.2% vs 14.3%), but these differences were not statistically significant in either group (p=0.535 for both). Vomiting was more frequent in the non-IMD-DILD group (20.4% vs. 4.8%), though no statistical significance was found (p=0.161). A total of 22 (29.3%) definitively discontinued treatment; 8 (38.1%) in the IMD-DILD group and 14 (25.9%) in the non-IMD-DILD group, without being statistically significant (p=0.299). Of these, 14 patients (58.3%) discontinued due adverse effects related to Nintedanib; 5 (62.5%) from the IMD-DILD group and 8 (64.3%) from the non-IMD-DILD group, with no statistically significant differences (p=0.979).
Conclusion: The tolerance and the occurrence of adverse effects involving drug withdrawal among patients treated with Nintedanib for IMD-DILD versus non-IMD-DILD was not different. However, it would be advisable to confirm these observations in a larger population.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Marina Molinari: None declared, Sara Salas: None declared, Luis Gómez Carrera Boehringer Ingelheim, GSK, María Torres Vargas Had participation in company sponsored speaker’s bureau from Boehringer Ingelheim, Carlos Javier Carpio Segura: None declared, Pablo Mariscal Aguilar: None declared, Gema Bonilla Boehringer Ingelheim, Bristol Myers Squibb, Glaxo Smith Kline, Nordic, UCB, Abbvie, Novartis, Sanofi, Horizon therapeutics, Bristol-Myers Squibb, Eugenio De Miguel Novartis, Abbvie, Pfizer, Janssen, Lilly, Abbvie, Novartis, Pfizer, MSD, BMS, UCB, Roche, Grunental, Janssen, Sanofi, Lilly, Abbvie, Novartis, Pfizer, Roche.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (