

Background: Chimeric Antigen Receptor (CAR) T-cell therapy is an emerging treatment for refractory rheumatological diseases, with potential for inducing drug-free remission in selected cases [1]. While its use in oncology has provided key insights since approval in 2017, its effects on fertility and pregnancy outcomes remain poorly understood. This is particularly important for rheumatology patients of reproductive age who may not have undergone prior gonadotoxic treatments.
Objectives: To systematically review available evidence on fertility and pregnancy outcomes following CAR T-cell therapy, and to evaluate current practices in patient counselling and fertility preservation.
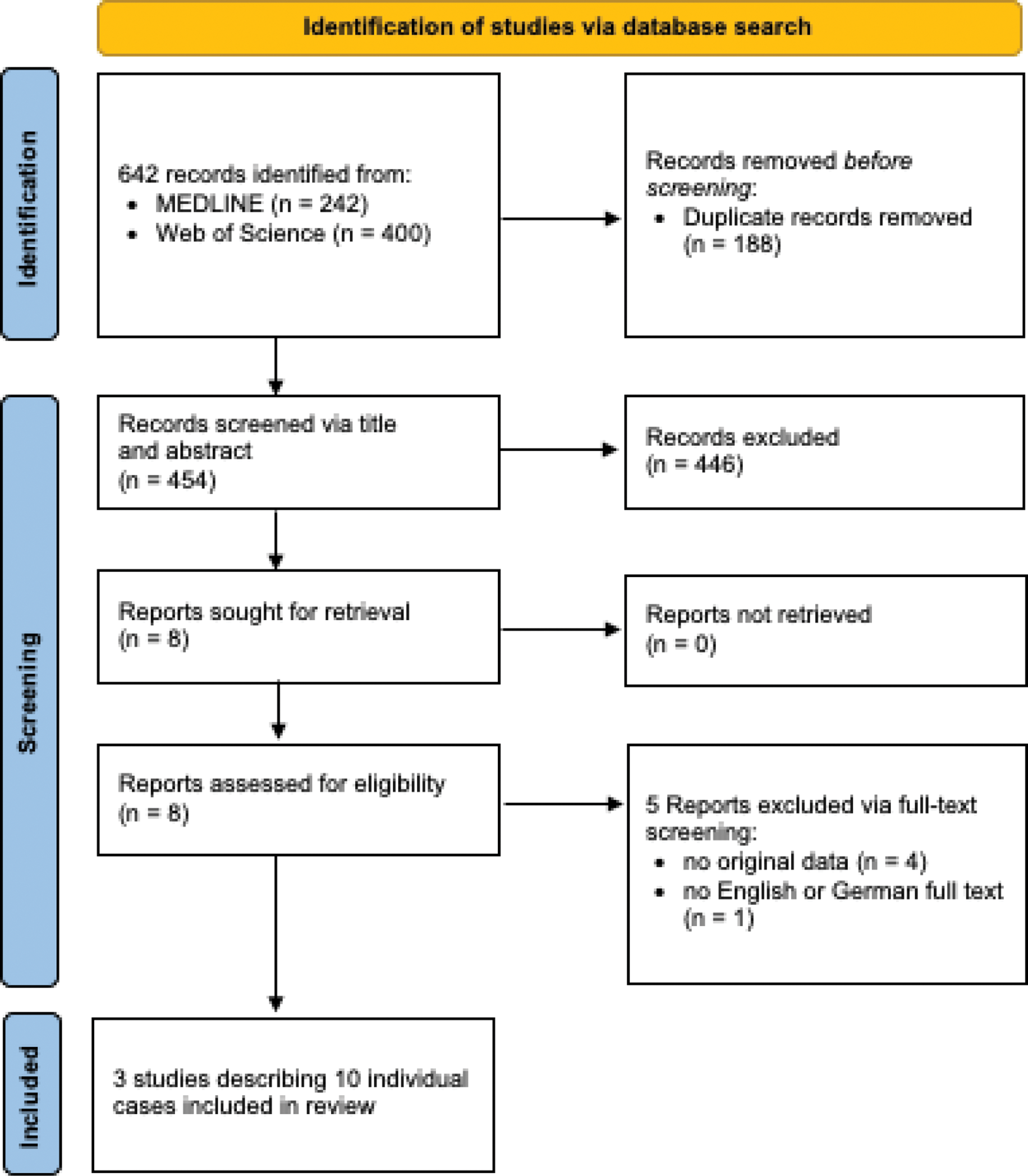
Methods: A systematic literature search was conducted using PubMed on January 13, 2024. Eligible studies included those reporting on fertility or pregnancy outcomes in men or women who underwent CAR T-cell therapy, as well as studies reporting on current practices in this field. Two independent reviewers screened and assessed relevant studies. Descriptive data were extracted and summarized.
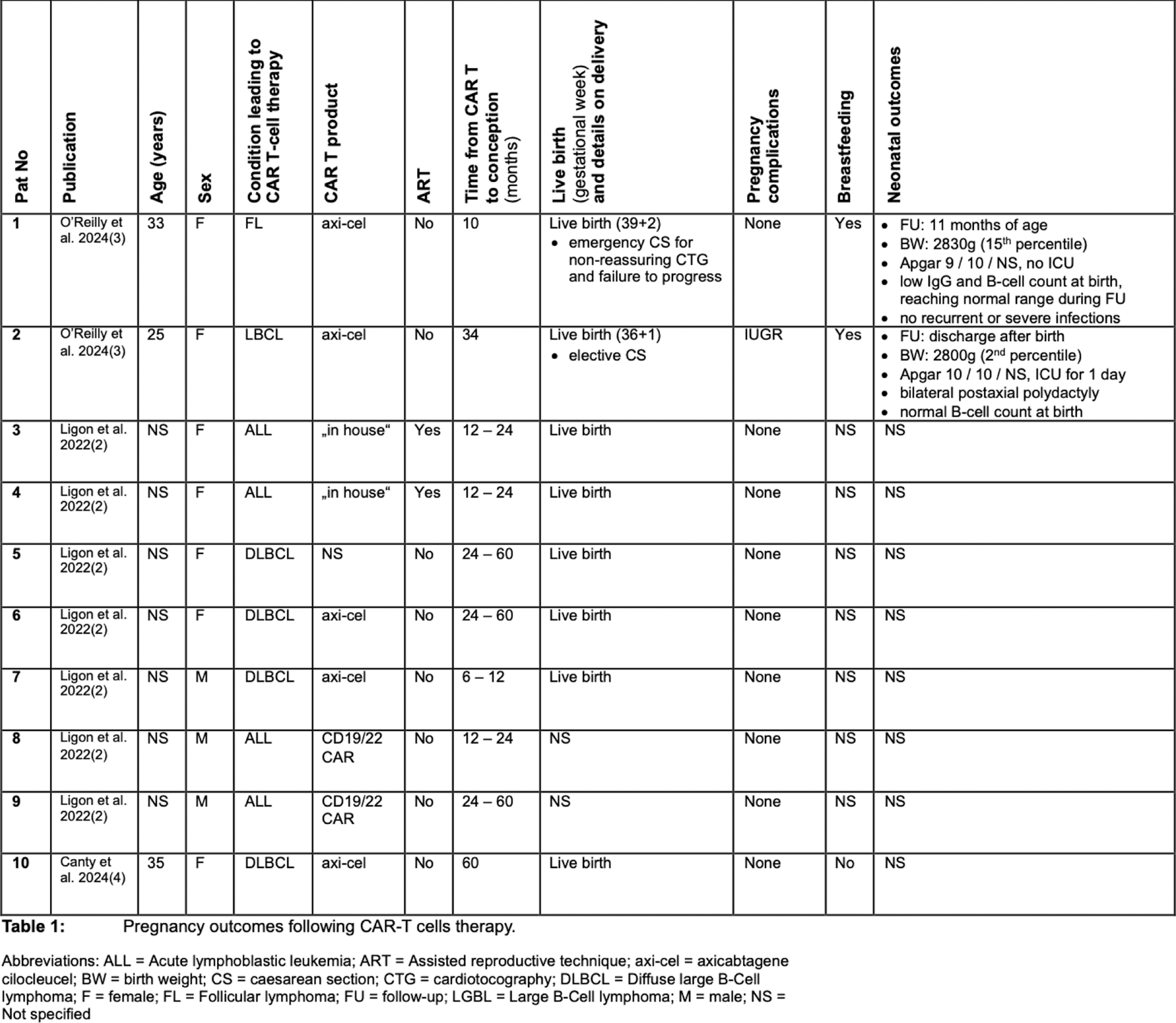
Results: Three studies (two full publications, one conference abstract) reported 10 pregnancies after CAR T-cell therapy of the mother (n = 7) or the father (n = 3) (see Table 1). Of the 10 pregnancies, 8 resulted in live births, while 2 had unknown outcomes. Pregnancy complications included one case of intrauterine growth restriction (IUGR) and one emergency cesarean section due to non-reassuring fetal heart tracing. Neonatal outcomes are only described in two cases with one polydactyly and one small-for-gestational-age child occurring. A survey of clinical practices revealed that nearly half of the centers provided limited counselling on fertility preservation or conception timing post-CAR T-cell therapy, and only a minority referred patients to fertility specialists [2].
Conclusion: The limited data highlight the need for more research on the reproductive health implications of CAR T-cell therapy in rheumatology. Unlike oncology, rheumatology patients often have not been exposed to prior gonadotoxic treatments, underscoring the importance of tailored guidelines and comprehensive counselling. Addressing these gaps is critical to support informed reproductive decision-making in this population.
REFERENCES: [1] DOI: 10.1056/NEJMoa2308917.
[2] DOI: 10.1016/j.jtct.2022.06.002.
[3] DOI: 10.1159/000542016.
[4] A HEALTHY PREGNANCY AND NEWBORN DESPITE PERSISTENCE OF MATERNAL CAR T-CELLS AFTER CANCER THERAPY; Canty, E. et al.; Annals of Allergy, Asthma & Immunology, Volume 133, Issue 6, S177 - S178.
Flow chart showing the selection process of the systematic review.
Acknowledgements: NIL.
Disclosure of Interesst: Phillip Kremer: None declared, Marie-Therese Holzer Abbvie, Boehringer Ingelheim, Rheumaakademie, Nikolas Ruffer: None declared, Ina Kötter Abbvie, Janssen, Lilly, Medac, Novartis, Sobi, GSK, Eusapharm und Boehringer, Martin Krusche Abbvie, UCB, Pfizer, Galapagos, Lilly, Boehringer-Ingelheim, Sobi, Novartis, BMS, Amgen, Sanofi, Janssen, Medac, GSK, FOMF, Abbvie, Boehringer-Ingelheim, Sobi, Novartis, Medac, Sobi, Novartis, Abbvie, Sanofi, Isabell Haase Abbvie, Alfasigma, AstraZeneca, Boehringer Ingelheim, Chugai, FOMF, GSK, Hexal, Johnson & Johnson, Lilly, Medac, Novartis, UCB, Abbvie, AstraZeneca, Boehringer Ingelheim, Medac.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (