

Background: Avacopan is a complement 5a receptor antagonist used as adjunctive treatment for granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA), which was Food and Drug Administration (FDA)-approved in 2021. Characteristics and outcomes among avacopan users in community-based practice have not been well characterized.
Objectives: Our objectives were to describe patients with GPA/MPA receiving avacopan in community rheumatology settings, identify their most commonly occurring disease manifestations, and explore treatment- and disease-related outcomes.
Methods: We examined data from the Excellence Network in RheumatoloGY (ENRGY), a U.S. community practice-based rheumatology research network that integrates electronic health record (EHR) data, lab results and claims data. Patients with a new prescription for avacopan were identified, and structured and unstructured data from the EHR were queried, manually reviewed centrally, and abstracted into electronic case report forms. Patients who were ≥18 years old with GPA, MPA, or undefined GPA/MPA with ≥6 months of avacopan use were included. Those with a baseline diagnosis of eosinophilic GPA (EGPA) or cancer (except non-melanoma skin cancer) were excluded. Patients were indexed on the date they initiated their first avacopan prescription and followed longitudinally until the earliest occurrence of loss to follow-up, death, end of avacopan prescription, or the end of the study period (September 2024). Duration of avacopan exposure was calculated from the index date to the last dose of the last prescription, and interruptions >4 weeks were noted but not included. Disease status at the time of avacopan initiation was described as new disease (≤6 months since diagnosis), relapsing disease (new disease activity in patients with previously resolved disease), or other (unclassified disease for >6 months without remission). Disease at the time of initiation was characterized as severe active disease if patients underwent concurrent induction with rituximab or cyclophosphamide and had pre-specified life- or organ-threatening manifestations. GPA/MPA-associated organ system manifestations were determined from the EHR at baseline and 1, 3, 6, and 12 months after index date. If present at baseline, disease manifestations were classified as worsened, persistent, improved, or resolved during follow up. Disease worsening, defined as new or worsening GPA/MPA manifestation(s) and escalation of glucocorticoid (GC) dose by prednisone-equivalent of ≥10mg/day, was evaluated during follow-up.
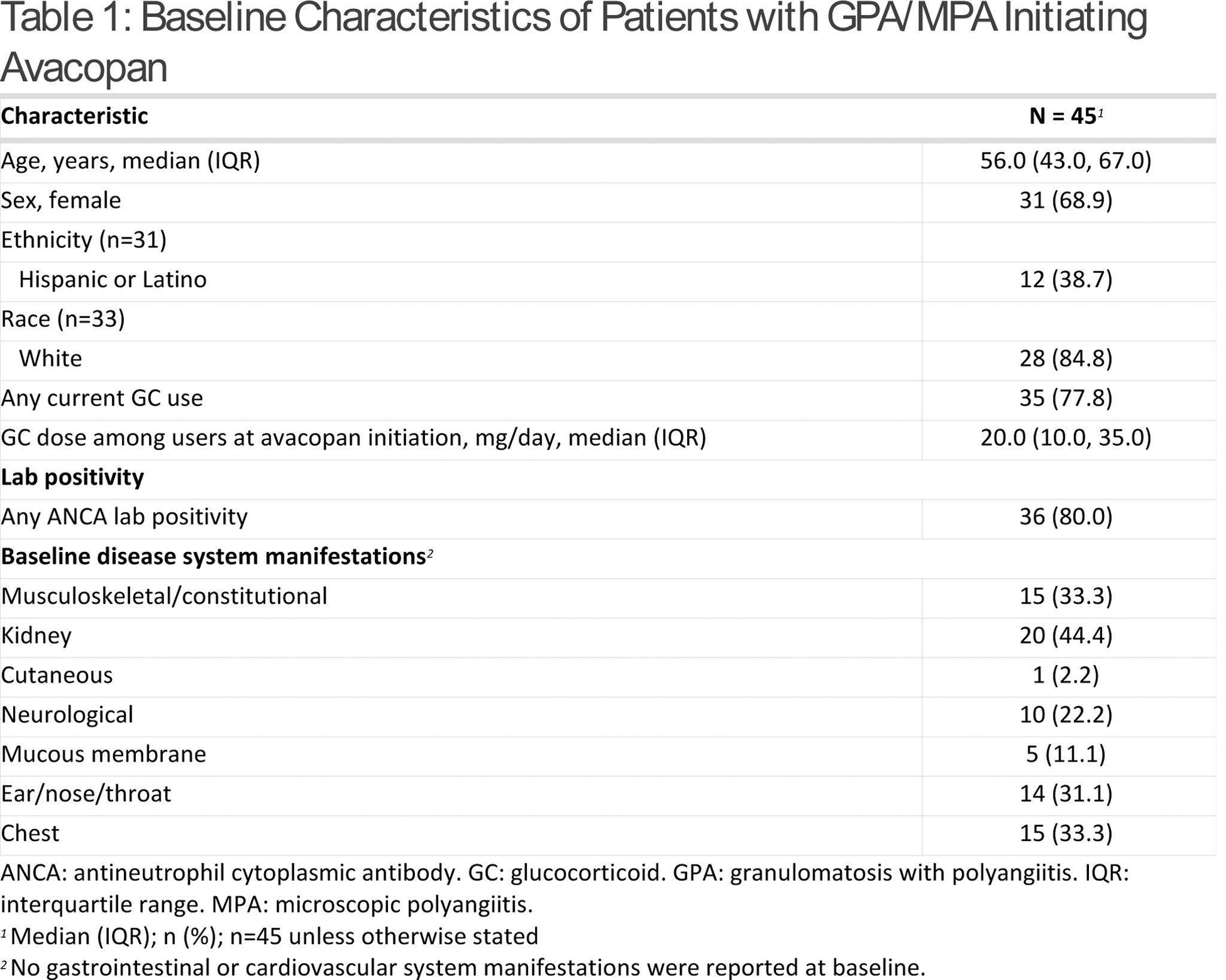
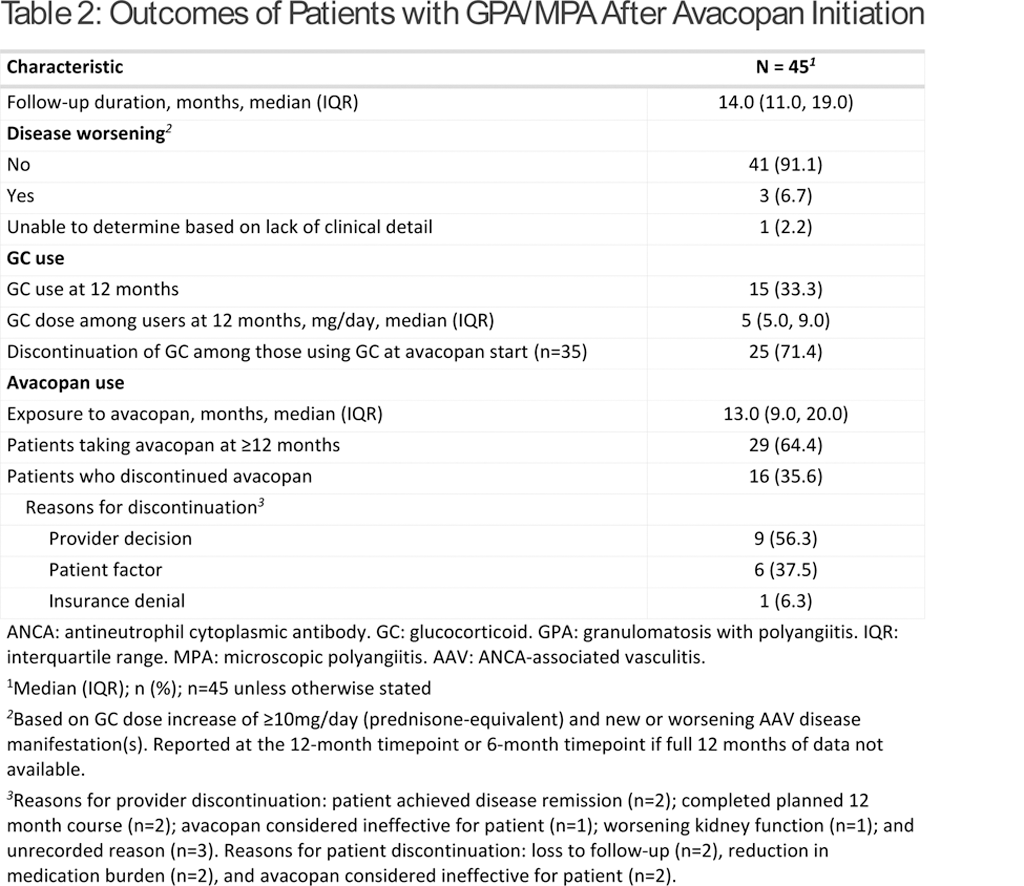
Results: From 10/19/21 to 12/04/23, 66 patients were prescribed avacopan. Seven patients were excluded as they had diagnoses of unspecified vasculitis, cryoglobulinemic vasculitis, EGPA, and/or rheumatoid vasculitis, and 14 were excluded because they did not initiate avacopan. Of the patients who initiated avacopan for GPA or MPA (n=45), median (interquartile range [IQR]) age was 56.0 (43.0, 67.0) years and 68.9% were female (Table 1). Among the patients with available race (n=33) and ethnicity (n=31) data, 84.8% were White and 38.7% were Hispanic. Patient diagnoses among the 45 patients who initiated avacopan included GPA (64.4%), MPA (22.2%), and undefined GPA/MPA (13.3%). Eighty percent of patients had a current or prior positive test for ANCA. Sixty percent of patients met defined criteria for severe active disease. At baseline, 51.1% of patients who initiated avacopan had relapsing disease, 42.2% had new disease, and 6.7% had unclassified disease. Median (IQR) prednisone-equivalent GC dose at the time of avacopan initiation was 20.0 (10.0, 35.0) mg/day. Rituximab was the most frequent concomitant medication among patients (62.2%). The most common organ system manifestations at baseline were kidney (44.4%), chest (33.3%), musculoskeletal/constitutional (33.3%), and ear/nose/throat (31.1%). Patients had a median (IQR) follow-up duration of 14.0 (11.0, 19.0) months from the date of avacopan initiation (Table 2). The median (IQR) duration of exposure to avacopan was 13.0 (9.0, 20.0) months. Three patients (6.7%) experienced disease worsening due to vasculitis during the follow-up period. Of the patients who were on GCs at avacopan initiation (n=35), 25 (71.4%) discontinued GCs within 12 months. Of those who were unable to discontinue GCs, 1 patient increased their GC dose due to worsening disease, 1 patient was on the dose utilized at avacopan initiation, 3 patients decreased but continued GCs at 12 months, and 5 patients did not have complete dosing information (due to stopping avacopan). Those who remained on GCs or started GCs during follow-up (n=15) had a median (IQR) dose of 5.0 (5.0, 9.0) mg/day at 12 months. Reasons for avacopan discontinuation at any point during the study period (n=16) are noted in Table 2.
Conclusion: Among patients with GPA/MPA on avacopan for at least 6 months in this real-world study, a majority (71%) on GCs at the time of avacopan initiation were able to discontinue GCs by 12 months, and the median dose of those who remained on GCs was low. Over 90% of patients had no disease worsening despite the significant reduction in GC use.
REFERENCES: NIL.
Acknowledgements: This study was supported by Amgen.
Disclosure of Interests: Brian Jaros: None declared, Emily Holladay: None declared, Amy Mudano: None declared, Yujie Su: None declared, Fenglong Xie: None declared, Shanette Daigle: None declared, Jeffrey R Curtis AbbVie, Amgen, Aqtual, BMS, GSK, Janssen, Lilly, Moderna, Novartis, Pfizer, Sanofi, Scipher, Setpoint, TNacity Blue Ocean, UCB (Consultant), AbbVie, Amgen, Aqtual, BMS, CorEvitas, GSK, Janssen, Lilly, Moderna, Novartis, Pfizer, Sanofi, Scipher, Setpoint, UCB (Grant/research support), Elizabeth Ibiloye Amgen (Shareholder), Amgen (Employee), Alexion AstraZeneca Rare Disease (Prior Employee), Darcy Trimpe Amgen, ChemoCentryx (Shareholder), Amgen (Employee), ChemoCentryx (Prior Employee), Sam Oh Amgen (Shareholder), Amgen (Employee), Zachary S. Wallace Amgen (Shareholder), Amgen (Employee), Alexion, Amgen, BioCryst, Boehringer Ingelheim, Horizon Therapeutics, MedPace, PPD, Visterra, Zenas (Consultant), Amgen, Sanofi (Grant/research support), Anisha B Dua Amgen, AbbVie, AstraZeneca, GSK, Novartis, Sanofi (Consultant), Amgen, Novartis (Grant/research support).
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (