

Background: Tumor necrosis factor-α inhibitors (TNFi) are the first biologic choice in most methotrexate inadequate responders with rheumatoid arthritis (RA). Yet, 30-80% of TNFi recipients fail to achieve an American College of Rheumatology 50% improvement (ACR50) at six months. A blood-based molecular signature response classifier (MSRC) was developed that detects gene expression patterns associated with inadequate response to TNFi [1]. Patients with a signal of inadequate response (SIR) were nine times less likely to attain ACR50 at six months when prescribed TNFi. Instead, when therapies aligned with MSRC results, outcomes improved [1–3]. Hispanics are the fastest growing ethnic minority in the United States, are under-represented in clinical trials and exhibit differences in RA clinical features and self-reported outcomes compared to non-Hispanic Whites.
Objectives: We evaluated the performance of MSRC in predicting SIR to TNFi in Hispanics with RA. We further explored whether MSRC informed advanced treatment selection and improved outcomes compared to standard of care.
Methods: We evaluated 314 self-identified Hispanic RA patients from a single practice with clinical disease activity index (CDAI)>10 on current treatment, in need to initiate or change biologic therapy. In an unmatched case-control design, we compared 108 MSRC-tested patients (cases) with 206 untested controls. Patients with SIR prescribed TNFi were classified as MSRC-misaligned, and patients with SIR prescribed a non-TNFi as well as those without SIR who received a TNFi or non-TNFi were considered MSRC-aligned. Main outcomes included CDAI low disease activity (CDAI-LDA, <10), minimal clinically important difference (CDAI-MCID), as well as low Routine Assessment of Patient Index Data-3 (RAPID3-LDA), low patient global assessment of disease (PtGA-LDA), and minimal pain visual analogue scale score (all ≤2 on a 0-10 scale). Secondary outcomes were absolute change from baseline to follow-up in CDAI and RAPID3 subscales (overall health, pain, and physical abilities). MSRC-aligned and MSRC-misaligned groups were compared on all outcomes in logistic regression models adjusted for age, ACPA positivity, fibromyalgia, follow-up duration, and the baseline value outcome. Linear regression models adjusted for the same covariates compared MSRC-aligned and MSRC-misaligned groups on absolute change in CDAI and RAPID3 subscales. Adjusted logistic regression models compared MSRC-aligned and untested controls on all outcomes. Inverse probability weighting was used to account for imbalance of baseline characteristics between the MSRC-aligned and untested control groups.
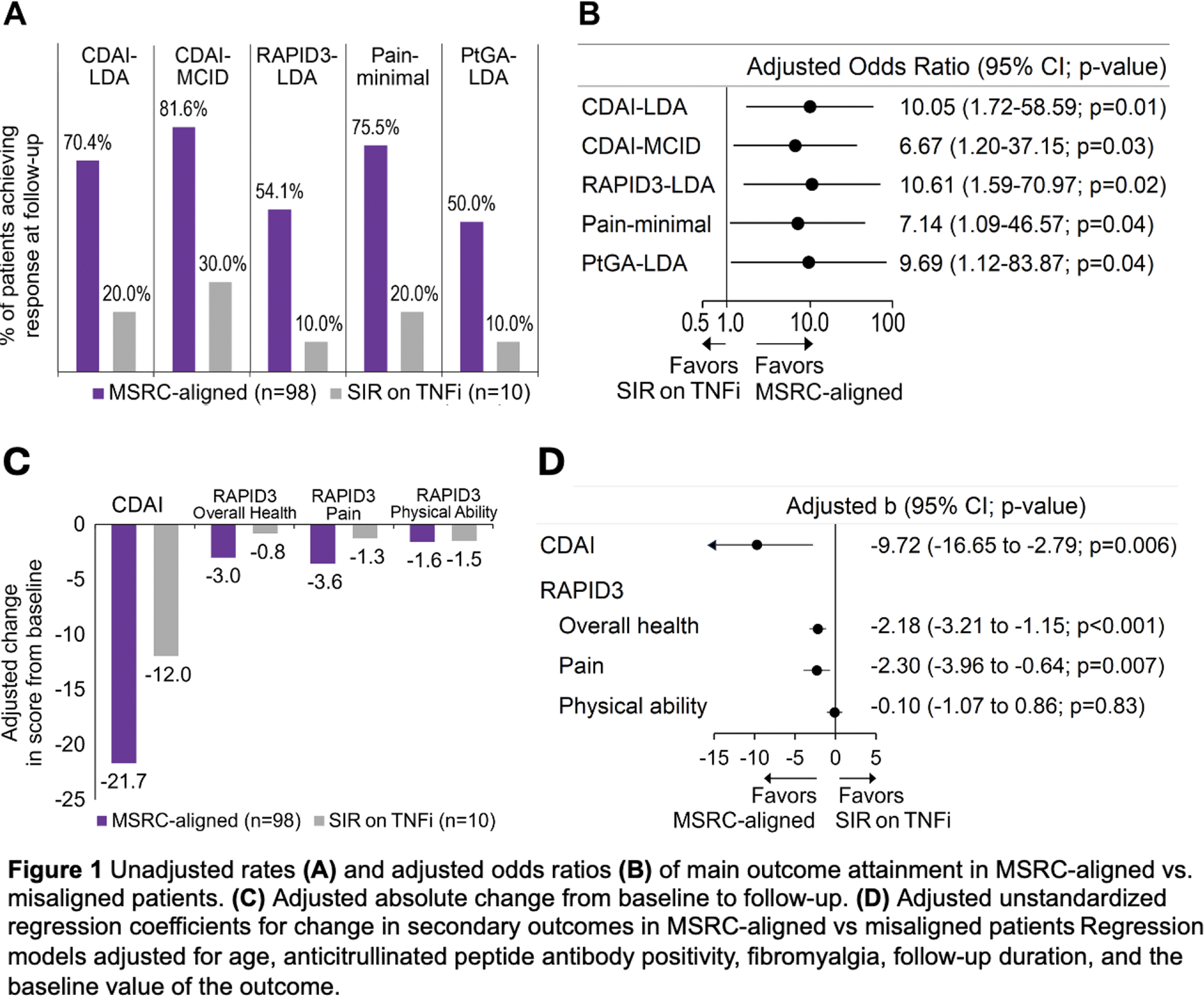
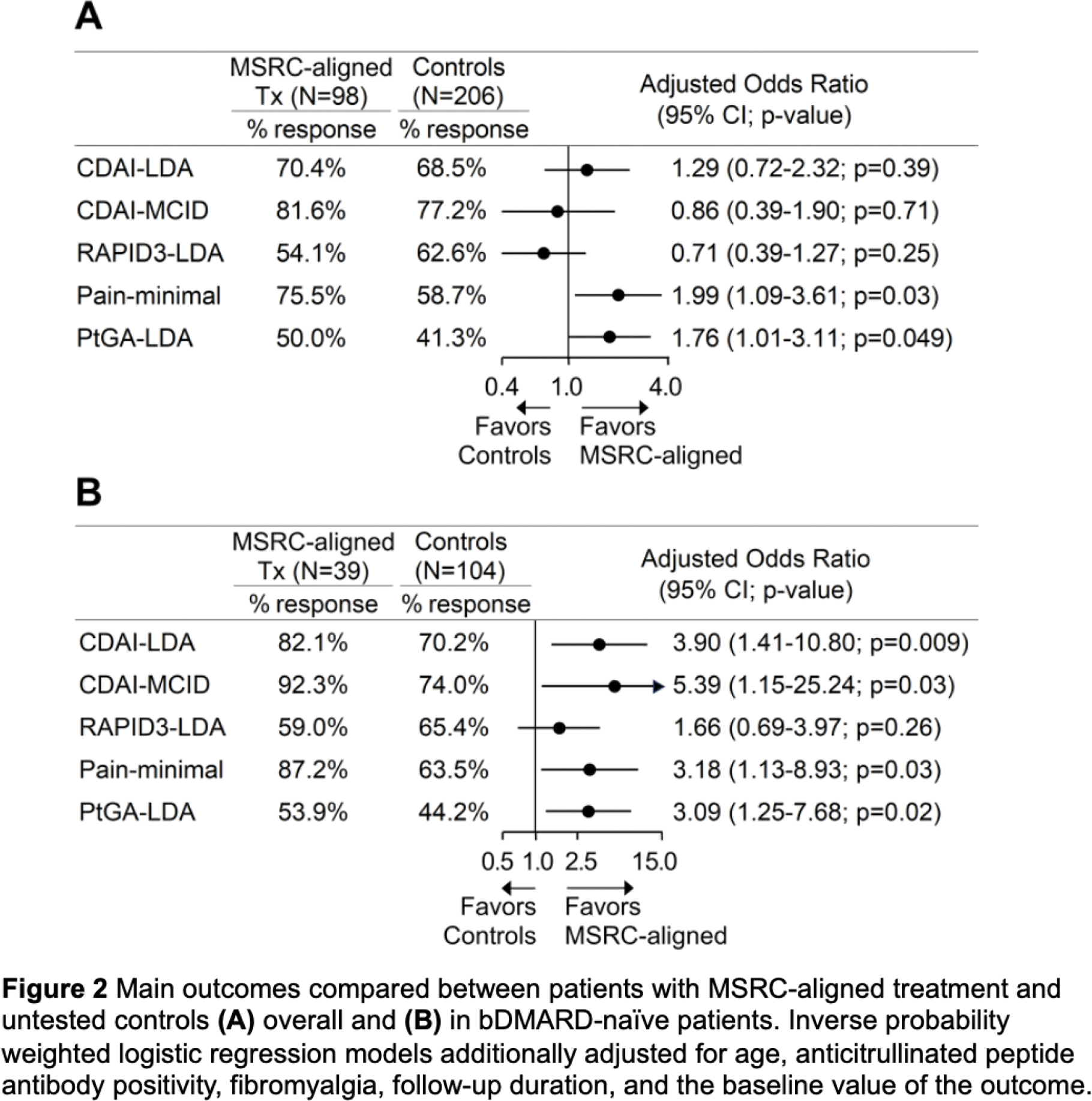
Results: Seventy (64.8%) MSRC-tested patients exhibited SIR; those received fewer TNFi versus controls (14.3% vs. 57.8%, adjusted odds ratio (aOR) 0.12 [95% confidence interval 0.06-0.25]). MSRC-aligned patients more frequently achieved CDAI-LDA, CDAI-MCID, RAPID3-LDA, PtGA-LDA and minimal pain (all p<0.04) compared to misaligned ones (Figure 1A and B). Compared to MSRC-misaligned patients, MSRC-aligned ones had greater decreases in CDAI and RAPID3 overall health and pain subscales (all p≤0.007) but not the RAPID3 physical abilities subscale (Figure 1C and D). Compared to controls, MSRC-aligned patients more commonly achieved PtGA-LDA and minimal pain (all p<0.049, Figure 2A). Among bDMARD-naïve participants, MSRC-aligned patients more frequently achieved CDAI-LDA, CDAI-MCID, PtGA-LDA, and minimal pain (all p<0.03) versus controls Figure 2B. In TNFi-treated patients, MSRC predicted SIR by CDAI-LDA with PPV=80.0% (95%CI 55.5-97.5%), sensitivity=50% (95%CI 29.9-75.4%), specificity=86.7% (95%CI 68.1-98.3%) and AUC=0.68 (0.53-0.84).
Conclusion: Two thirds of Hispanic patients exhibited SIR to TNFi. MSRC-aligned therapy improved outcomes versus MSRC-misaligned group and untested controls, mostly among bDMARD-naïve patients. Therefore, broader adoption of MSRC testing may alter treatment paradigms and optimize clinical outcomes.
REFERENCES: [1] Cohen S et al. Rheumatol Ther 2021;8:1159–76.
[2] Curtis JR et al. Expert Rev Mol Diagn 2022:1–10.
[3] Strand V et al. Expert Opin Biol Ther 2022;22:801–7.
Acknowledgements: NIL.
Disclosure of Interests: George Karpouzas Scipher, Janssen, Scipher, Pfizer, Miguel Rodriguez Scipher, Viet Bui: None declared, Sarah Ormseth: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (