

Background: Eosinophilic granulomatosis with polyangiitis (EGPA) is a systemic necrotizing vasculitic disease affecting multiple organs, resulting in high disease burden [1, 2]. The reported prevalence of EGPA varies globally, from 2.0 to 30.4 cases per million people from 1994–2013, with a marked increase in Japan from 4.2 cases per million in 2005 to 58.6 per million in 2020 [3, 4]. EGPA is typically treated with oral corticosteroids (OCS), but long-term use is linked to adverse events (AEs) [5]. Mepolizumab was approved in Japan for adults with EGPA inadequately treated with other therapies, based on the MIRRA study (NCT02020889) [6]. Mepolizumab increased the percentage of patients in remission for ≥24 weeks (28% vs 3%) and 44% of patients had a reduced average daily OCS dose of ≤4.0 mg/day [6]. Real-world data in Japan also reported a 71% reduction in average daily OCS dose after ≥192 weeks of mepolizumab treatment [7]. However, research on the effectiveness of mepolizumab in certain subgroups of patients with EGPA remains limited.
Objectives: To assess the long-term safety and effectiveness of mepolizumab in key EGPA subgroups from the MARS study, including by disease duration, OCS dose, EGPA relapse, anti-neutrophil cytoplasm antibodies (ANCA) status, and immunosuppressive therapy use.
Methods: MARS (NCT04551989) is a 96-week, single-arm observational study that assessed long-term safety and effectiveness of mepolizumab in patients with EGPA in Japan. Patients had previously completed a Japan post-marketing surveillance study (NCT03557060, ≥96 weeks of mepolizumab treatment, 300 mg subcutaneous every four weeks, before study entry [baseline]) and continued receiving mepolizumab for another 96 weeks [7]. Data were collected every 12 weeks from baseline to Week 96 (observation period), with reference data collected ≤48 weeks before the first mepolizumab dose (retrospective period). Outcomes included AEs, OCS dose, clinical symptoms, and EGPA relapse rates.
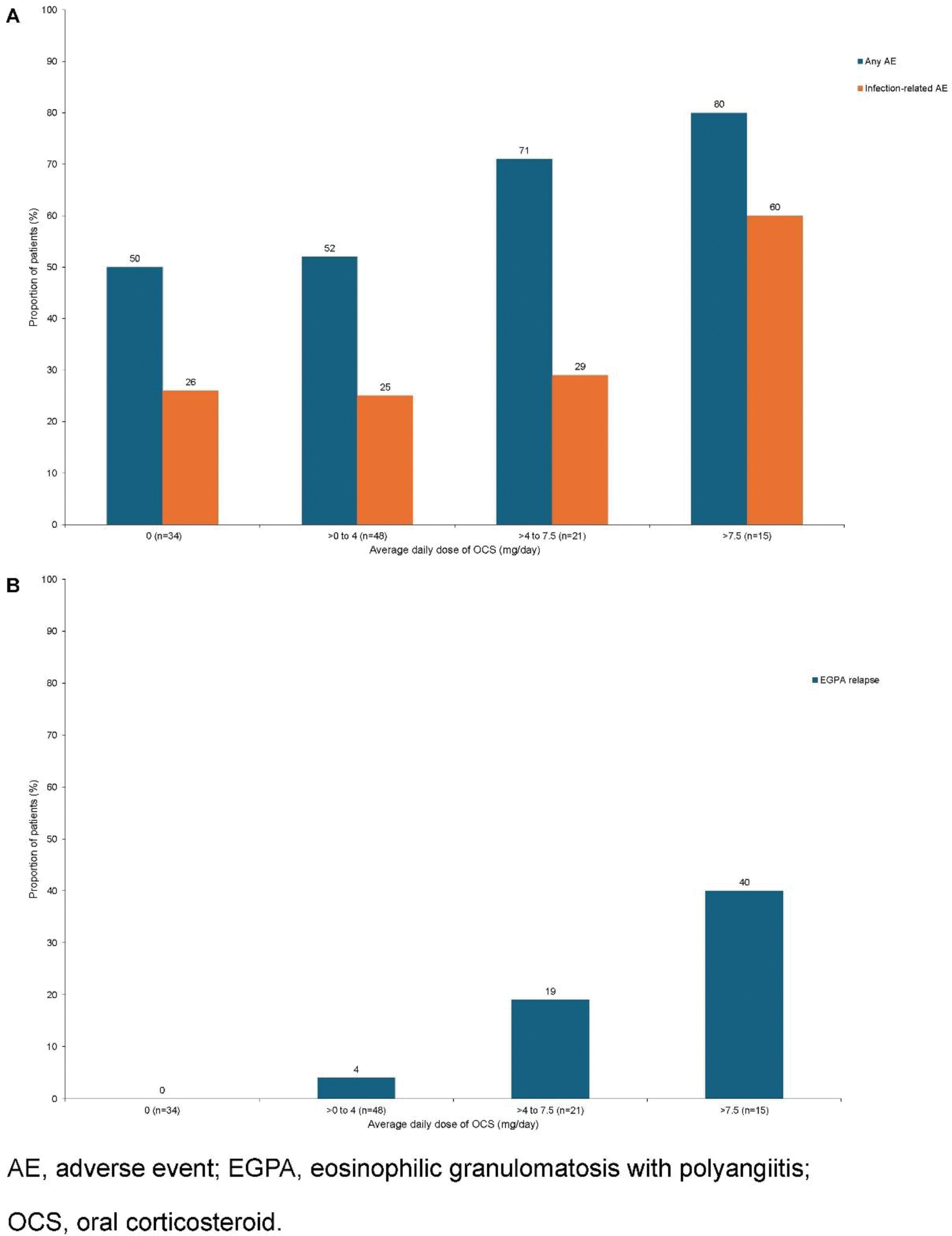
Results: A total of 118 patients were enrolled; 47% received mepolizumab within the first year of diagnosis. In patients with a medical history of EGPA ≥10 years, 88% reported AEs, while 50–59% of patients with shorter EGPA medical histories (≤1, >1 to 2, >2 to 5, and >5 to 10 years) reported AEs. However, the number of patients in the ≥10 years group was small (n=8). Medical history duration did not impact the presence of EGPA symptoms, with 67–100% of patients reporting no clinical symptoms at Week 96, except for sensory and motor nervous system symptoms (30–86%). From the retrospective period to Weeks 93–96, mepolizumab reduced the OCS dose for patients with medical histories of ≤1 to ≤10 years (~69–100%), but those with >10 years had lower reductions (~10%). Most patients remained relapse-free (83–100%). The proportion of patients on an OCS dose >7.5 mg/day decreased from 44% in the retrospective period to 11% at baseline and 8% by the end of the observation period. The proportion receiving 0 mg/day OCS dose increased (8%, 31%, and 36%, respectively). There was a higher proportion of AEs, including infections and fractures, with increasing OCS doses (Figure 1A). At Week 96, more patients on higher OCS doses (>7.5 mg/day) reported clinical symptoms (≤62%) versus lower doses (≤44%). Relapse rates also increased with higher OCS doses, with no relapses in patients receiving 0 mg/day (Figure 1B). Overall, 90% of patients did not experience a relapse. Those with a relapse had more AEs than those without (any AE, 83% vs 56%; infection-related, 58% vs 27%, respectively). Those without relapse had stable symptoms, with 85–100% reporting no clinical symptoms at Week 96, except for sensory and motor nervous system symptoms (56% and 69%, respectively). An 85% reduction in the median average daily OCS dose was also observed from the retrospective period to Weeks 93–96. Forty-one patients had ANCA status (37 negative; 4 positive). Of the ANCA negative subgroup (n=37), 54% reported AEs (24% infection-related AEs). Most (88–100%) reported no clinical symptoms at Week 96, except for sensory and motor nervous system symptoms (44% and 66%, respectively). From the retrospective period to Weeks 93–96, the median average daily OCS dose decreased from 7.0 to 0 mg/day. At Week 96, 95% of patients reported no relapses. Among patients who had not received immunosuppressive therapy at baseline (71%), AEs occurred more frequently (any AE, 64%; infection-related, 32%) than in those who had received therapy (any AE, 44%; infection-related, 26%). In both groups, 80–100% of patients reported no clinical symptoms at Week 96, except for sensory and motor nervous system symptoms (54–73%). The median average daily OCS doses were ~1–3 mg/day over the observation period; 11% of patients reported relapses without immunosuppressive therapy and 9% with.
Conclusion: Patients with EGPA disease receiving high OCS dose (>7.5 mg/day) reported more AEs, more symptoms, and higher relapse rates than lower doses (<7.5 mg/day). Despite the duration of EGPA prior to mepolizumab treatment, ANCA-negative status, or immunosuppressant use at baseline, mepolizumab remains effective, though its impact on OCS reduction varied across subgroups. For relapse-free patients, continued mepolizumab use led to fewer AEs, lower OCS doses, and improved symptoms over time.
REFERENCES: [1] White J, et al. (2023) Autoimmun Rev. 22:103219.
[2] Khoury P, et al. (2023) Mayo Clin Proc. 98:1054–70.
[3] Jakes RW, et al. (2021) Clin Rheumatol. 1–8.
[4] Sada K, et al. (2024) Mod Rheumatol. 34:988–98.
[5] Doubelt I, et al. (2021) ACR Open Rheumatol. 3:404–12.
[6] Wechsler ME, et al. (2017) N Engl J Med. 376:1921–32.
[7] Ishii T, et al. (2024) Mod Rheumatol. Roae100.
Acknowledgements: This study was funded by GSK (study 213684).
Disclosure of Interests: Tomonori Ishii TI is employed, by Tohoku University Hospital, who received funding from GSK for this study, Hideaki Kunishige HK holds financial equities in GSK, and is employed by GSK, Mitsuhiro Yoshida MY holds financial equities in GSK, and is employed by GSK, Etsuko Hayashi EH holds financial equities in GSK, and is employed by GSK, Masaki Komatsubara MK holds financial equities in GSK, and is employed by GSK, Rafael Alfonso-Cristancho RA-C holds financial equities in GSK, and is employed by GSK, Peter Howarth PH holds financial equities in GSK, and is employed by GSK.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (