

Background: Ankylosing spondylitis (AS) severely impacts individuals by limiting mobility, increasing pain, and causing fatigue, substantially impairing daily functioning. Traditional methods for measuring disease activity and physical function in trials and clinical practice rely on discrete, largely subjective, single-point assessments that may not fully capture disease fluctuations. Wearable technology offers a method for continuous and passive monitoring of human behaviour in a free-living environment and has the potential to enhance the evaluation of treatment effectiveness in AS [1].
Objectives: To explore the impact of secukinumab (SEC) 150mg treatment on device-measured physical activity (PA) in patients with ankylosing spondylitis and to investigate the association between clinical and fatigue responder statuses on group-level physical activity trends.
Methods: ASLeap was a randomized, double-blind, parallel-group, multicentre, phase 4 trial comparing secukinumab (SEC) 300 mg vs 150 mg in participants who had an inadequate response following a 16-week open-label treatment arm where all participants received SEC 150 mg [2]. Daily physical activity, measured as total activity counts per day, was monitored using a Philips Actiwatch Spectrum wrist-worn device during a 14-day pre-treatment screening phase and throughout the treatment period (day 0 to week 16). A compliant day of device wear was defined as continuous wear over 24 hours. Participants with ≤70% total compliant days were excluded. Ankylosing Spondylitis Disease Activity Score (ASDAS) responders were defined as participants who achieved an improvement in ASDAS-CRP score of ≥2.0 between baseline and week 16. Fatigue responders were defined based on an improvement of ≥8 in FACIT-Fatigue score [3]. The effect of SEC on daily PA was assessed using generalized additive models (GAMs). A smoothing parameter for study day was included to model the overall trends in PA. A binary dummy variable for pre- and post-treatment initiation was including to model the independent slopes of PA in response to SEC. We analysed PA trends stratified by ASDAS and FACIT-fatigue responder groups, incorporating interaction terms to assess how responder status modulated the treatment effect on daily PA levels. GAMs were adjusted for covariates including sex, age, and season with a subject-level random effect added.
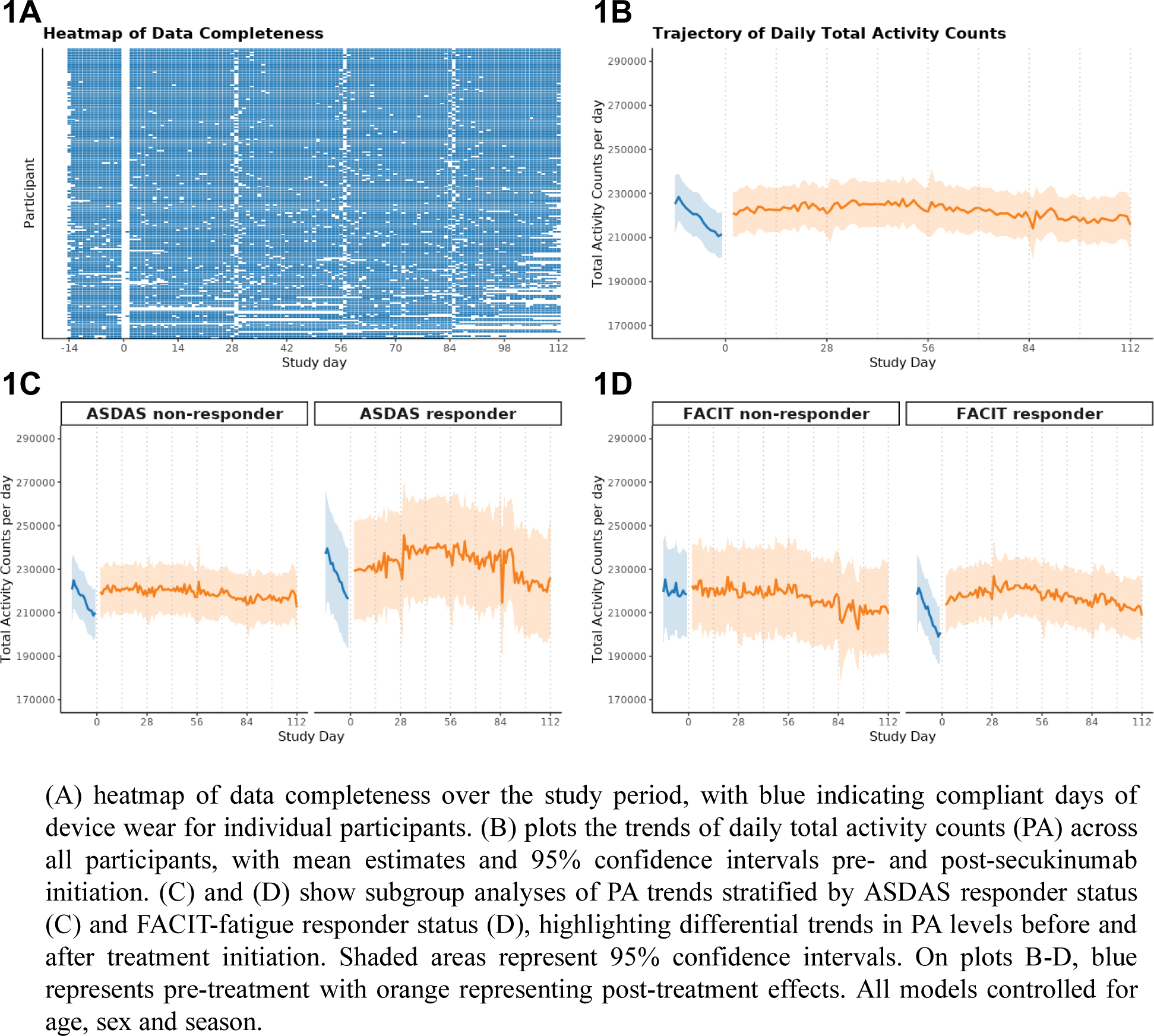
Results: Participants (n=185) had a mean age of 48.8 years (standard deviation (SD) 15.3). Participants had a mean disease duration of 4.1 years (SD 9.1), with the majority being TNF-α inhibitor naïve (68.3%) (Table 1). Baseline ASDAS-CRP scores were significantly higher in non-responders compared to responders (4.3 vs. 3.6, p<0.001), while baseline FACIT-fatigue scores were similar between groups. Device compliance was high, with participants wearing devices for a mean of 112.9 days (SD 9.6), and baseline total daily activity counts per day were comparable between ASDAS responders and non-responders (227,309 vs. 215,691, p = 0.38) (Table 1). Treatment with SEC was associated with a higher daily PA from a baseline of 220,130 counts per day of 4153 counts per day (p = 0.001). In the pre-treatment window, PA levels declined significantly over time, with a negative linear slope (p < 0.001). Following treatment initiation, the trend for PA demonstrated an approximately flat slope, indicating stabilisation of PA levels at the group level (p = 0.009), (Figure 1B). For ASDAS non-responders, pre-treatment PA levels declined significantly over time (p < 0.001), but after treatment initiation, stabilisation of PA levels was observed (p = 0.004) (Figure 1C). In contrast, for ASDAS responders, post-treatment PA levels demonstrated modest non-linear positive trends over time (p = 0.03). For FACIT non-responders, the smoothing term for pre-treatment PA trends was not significant, indicating no meaningful change in PA levels over time prior to treatment initiation. Post-treatment, PA levels showed no significant improvement or non-linear trends (p = 0.64) (Figure 1D). In contrast, for FACIT responders, the smoothing term for post-treatment trends was significant (p = 0.003), indicating a modest non-linear increase in daily PA over time following treatment initiation. Pre-treatment PA trends also showed significant decline over time (p < 0.001), highlighting distinct PA trajectories compared to non-responders.
Conclusion: In this exploratory analysis, SEC treatment was associated with higher device-measured physical activity (PA) levels, with differences in trends observed by responder status. Whilst these findings are interesting, a comparative RCT is needed to confirm treatment group differences. These findings highlight the potential for wearable devices to capture treatment effects using novel patient-centric measures in AS.
REFERENCES: [1] McGagh D, et al. Lancet Rheumatology. 2024 Jul 29.
[2] Deodhar A, et al. Rheumatology. 2024 Aug 12:keae432.
[3] Cella D, et al. Journal of Patient-Reported Outcomes. 2024 Aug 12;8(1):92.
Table 1. Demographics and baseline disease characteristics of patients receiving SEC 150 mg.
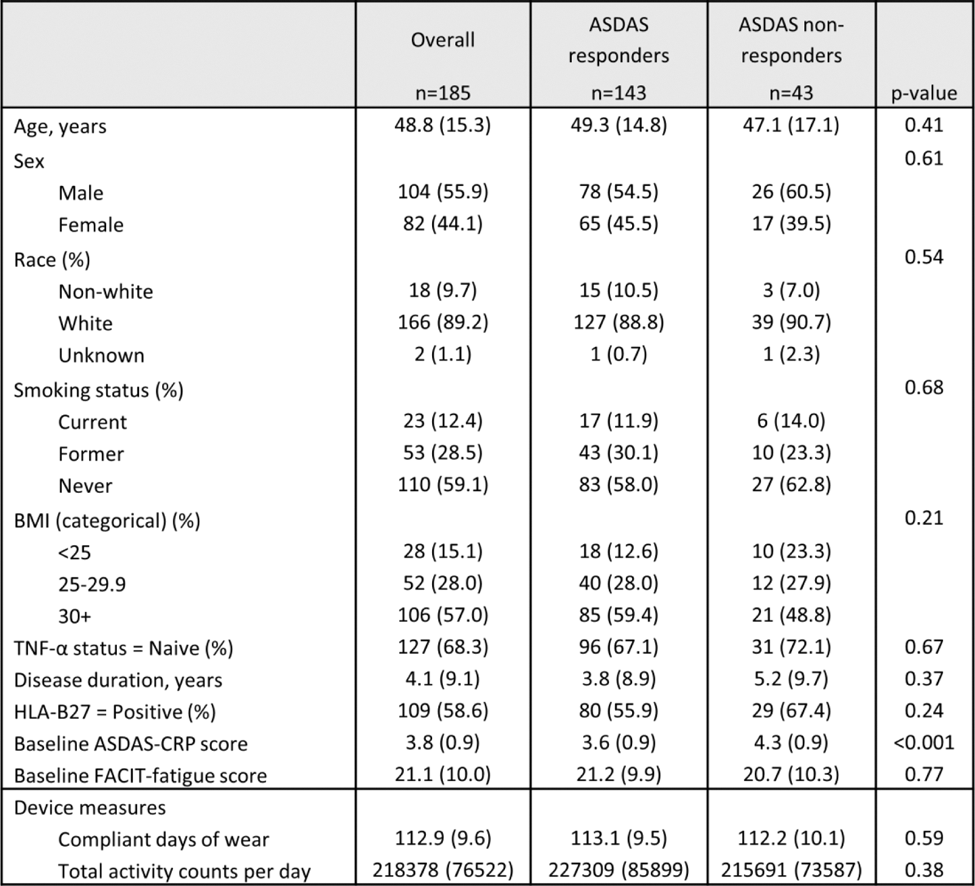
Heatmap of data completeness and trends in device-measured physical activity following treatment with secukinumab in the ASLeap study.
Acknowledgements: NIL.
Disclosure of Interests: Dylan McGagh Received funding from Novartis to permit secondary analysis of ASLeap, Linda Grinnell-Merrick Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA, Adam Winseck Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA, Thomas E. Nichols Perspectum Ltd, Novartis, Aiden Doherty GSK, Laura C. Coates AbbVie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly and Company, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, Prothena, Sun Pharma, UCB Pharma, Eli Lilly and Company
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (