

Background: Canakinumab (CAN), a monoclonal antibody targeting IL-1β, has shown significant efficacy in various autoinflammatory diseases. However, real-world evidence, especially in familial Mediterranean fever (FMF) and other IL-1β-driven conditions, is limited. This study aims to evaluate Canakinumab’s therapeutic impact, safety, and dosing adaptations in a tertiary rheumatology setting.
Objectives: This study aims to evaluate the usage patterns, clinical outcomes, and safety profile of CAN in a tertiary rheumatology clinic.
Methods: This retrospective, single-center study included 54 patients treated with CAN between May 2020 and September 2024. A total of 54 patients were categorized into MEFV-positive FMF (n=42), MEFV-negative FMF (n=7), non-FMF autoinflammatory diseases (n=2), and Adult-Onset Still’s Disease (AOSD) (n=3). Clinical and laboratory data, including treatment duration, response rates, and adverse events, were extracted from patient records.
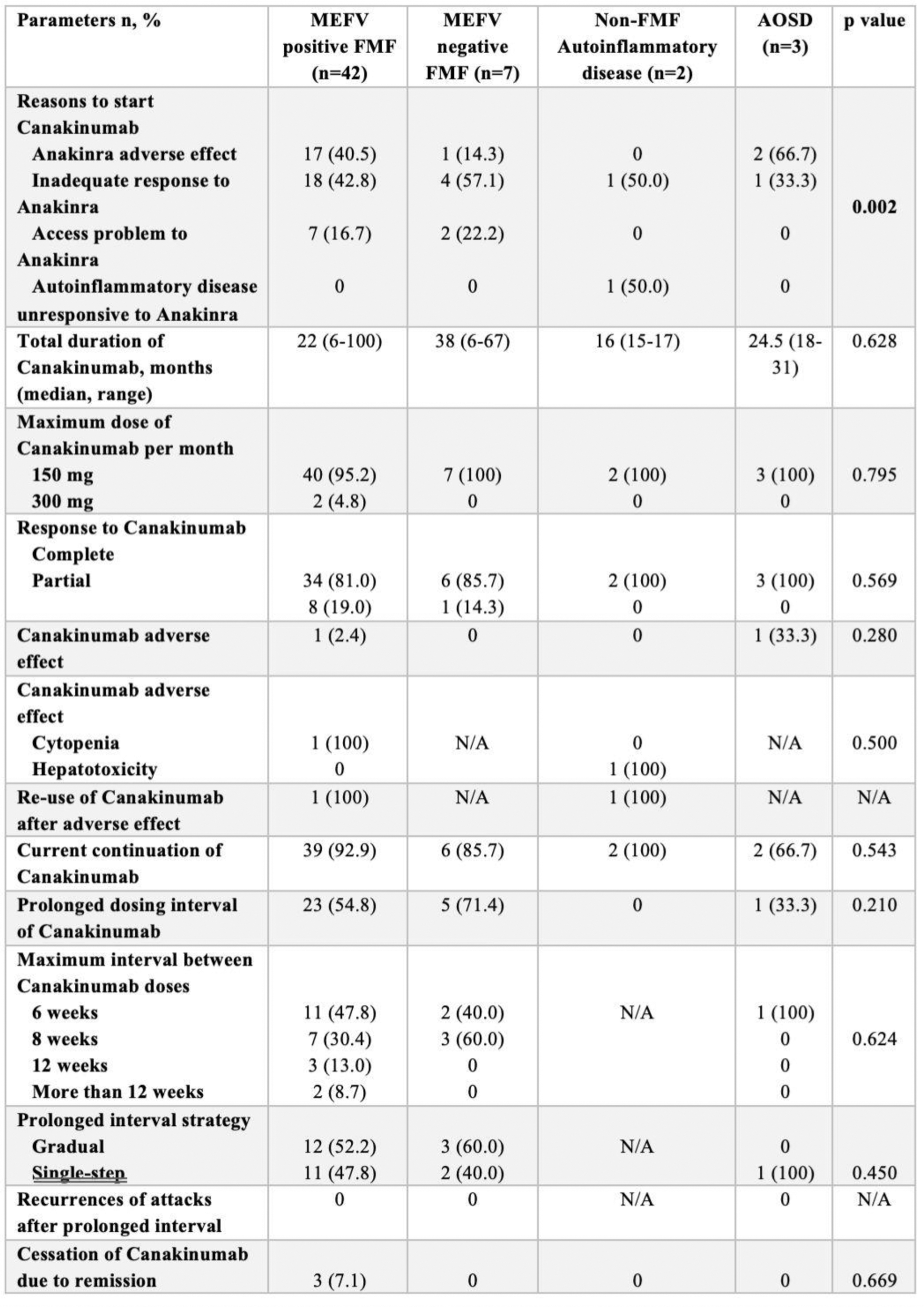
Results: Demographic and clinical characteristics are presented in Table 1. MEFV-positive FMF patients were diagnosed significantly earlier (median: 19.5 years) compared to MEFV-negative patients (median: 35 years, p=0.012), and familial FMF history was markedly higher among MEFV-positive patients (59.5% vs. 14.3%, p=0.033). CAN was initiated primarily due to inadequate responses to Anakinra (42.8%) and adverse reactions to Anakinra (40.5%) in MEFV-positive FMF patients (Table 2). The median treatment duration with CAN was 22 months, with 95.2% of patients receiving a maximum dose of 150 mg/month. No significant differences in CAN-related parameters were observed between MEFV-positive and MEFV-negative FMF groups, underscoring its efficacy across genotypes. Canakinumab demonstrated robust clinical efficacy, achieving complete remission in 81.0% of MEFV-positive FMF patients and partial responses in 19.0% (Table 2). Treatment significantly reduced attack duration (median 4 to 2 days, p<0.001) and attack frequency, with a notable increase in attack-free intervals (p<0.001). Systemic inflammation markers, including CRP, ESR, WBC, and neutrophil counts, were significantly improved (for all p<0.001). Renal function, assessed via uPCR, also showed a modest but significant reduction (p=0.010). Adverse events were minimal, with only one reversible cytopenia reported. Efficacy and safety were consistent across MEFV-positive and MEFV-negative groups. Prolonged dosing intervals were successfully implemented in 54.8% of MEFV-positive patients, with intervals extended up to 12 weeks in 47.8%. These extensions were achieved through both gradual (52.2%) and single-step (47.8%) strategies, without compromising efficacy or increasing recurrence risk.
Conclusion: This highlights the clinical utility of CAN in managing refractory FMF and other autoinflammatory conditions. CAN demonstrates robust efficacy and safety, with flexible dosing strategies offering additional benefits. These findings support CAN’s role as a cornerstone therapy in autoinflammatory diseease field of rheumatology practice.
REFERENCES: NIL.
Table 1. Baseline Demographics, Clinical Characteristics, and Previous Treatments in Study Groups.
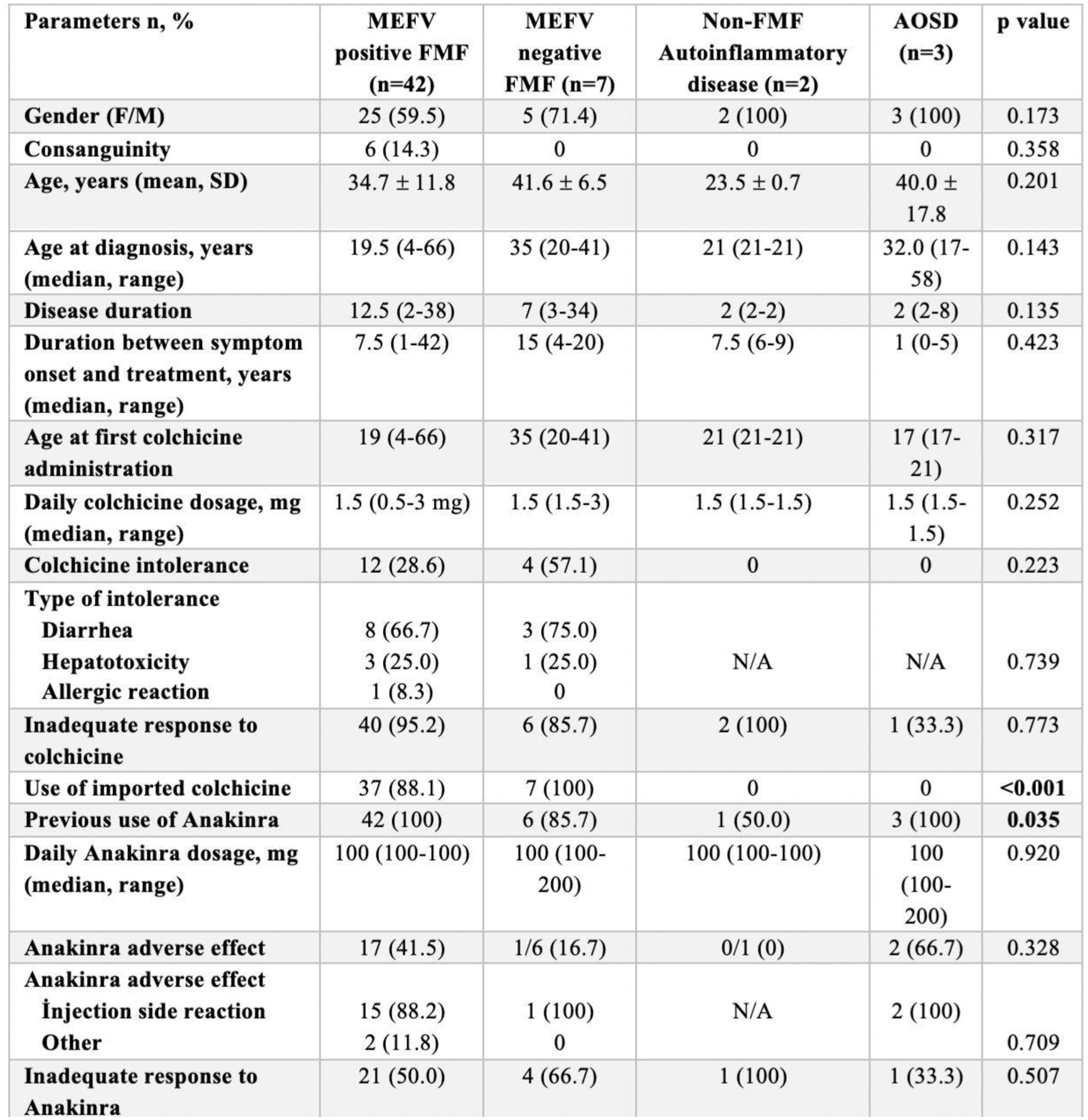
Table 2. Canakinumab Treatment Parameters and Outcomes in Study Groups.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (