

Background: Anti-U1-Ribonucleoprotein (RNP) is an autoantibody include into extractable nuclear antigens (ENAs). It targets an intracellular protein involved in the maturation of messenger ribonucleic acid. Anti-RNP has been associated with different connective tissue diseases (CTD), such as systemic lupus erythematosus (SLE), systemic sclerosis, mixed connective tissue disease (MCTD), and idiopathic inflammatory myopathies, among others [1]. RNP antibodies may coexist with other anti-ENAs or appear as the sole specificity [2]. Anti-ENAs are traditionally detected using ELISA and chemiluminescence assays, but emerging technologies such as particle-based multi-analyte technology (PMAT) provide simultaneous detection and semi quantification of autoantibodies [3].
Objectives: To assess a ) the association between isolated anti-RNP and CTD as well as clinical and laboratory findings, and b ) the relationship with antibody quantitation titres.
Methods: We reviewed all anti-ENA tests performed by PMAT in a referral hospital between March 2022 and April 2023. PMAT (Aptiva, Werfen, San Diego, USA) used CTD Essential Reagent. We identify patients with isolated positive anti-U1-RNP antibodies. A level over 5 fluorescence units (FLU) was considered positive. Following manufacturer instructions, patients were stratified into three groups based on anti-RNP titres: low (5-20 FLU), medium (20-80 FLU), and high (>80 FLU). Subsequently, medical records were examined to determine the presence of CTDs accordingly to international classification criteria. Additionally, demographic data, clinical features, laboratory findings, and the number of outpatient rheumatology visits in the past year were recorded.
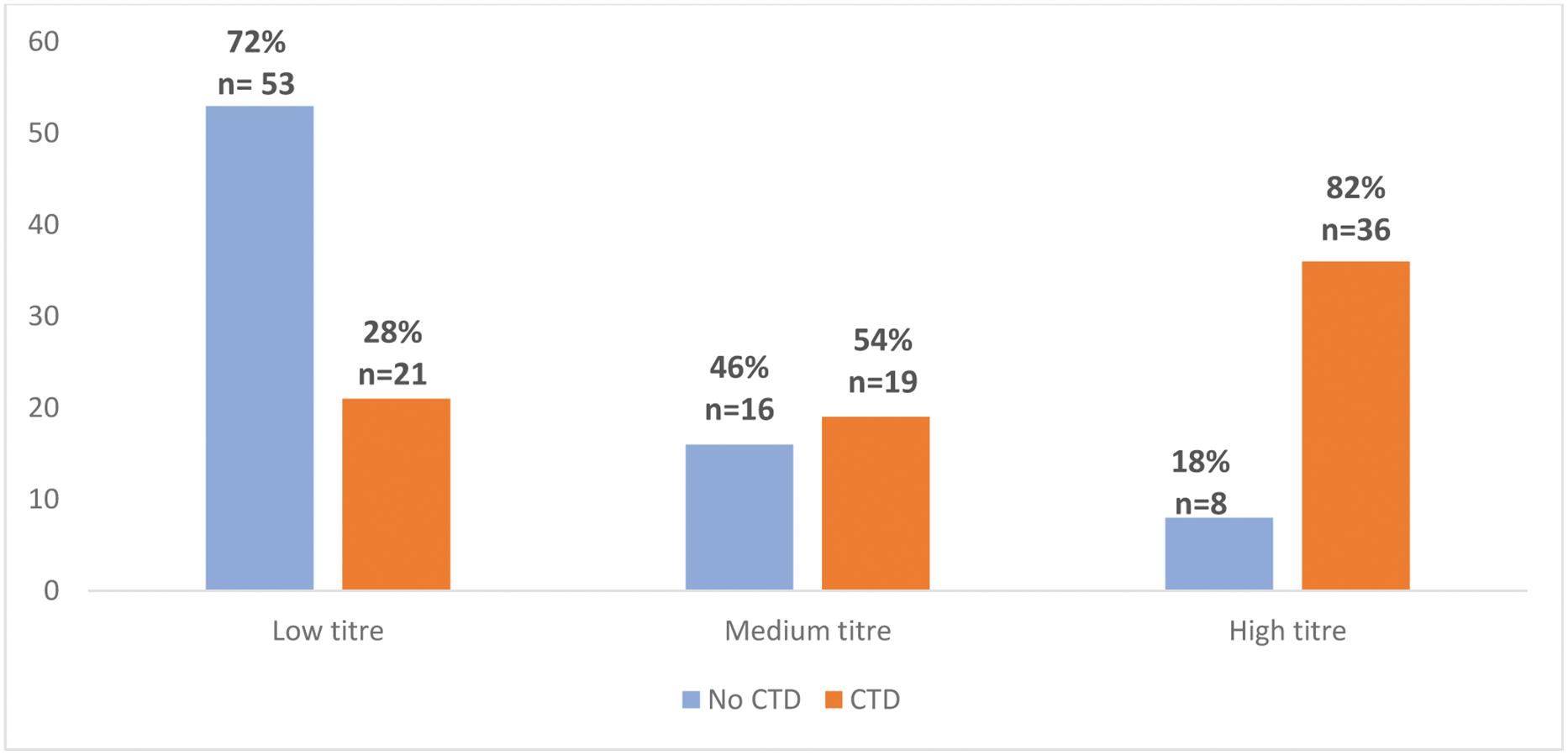
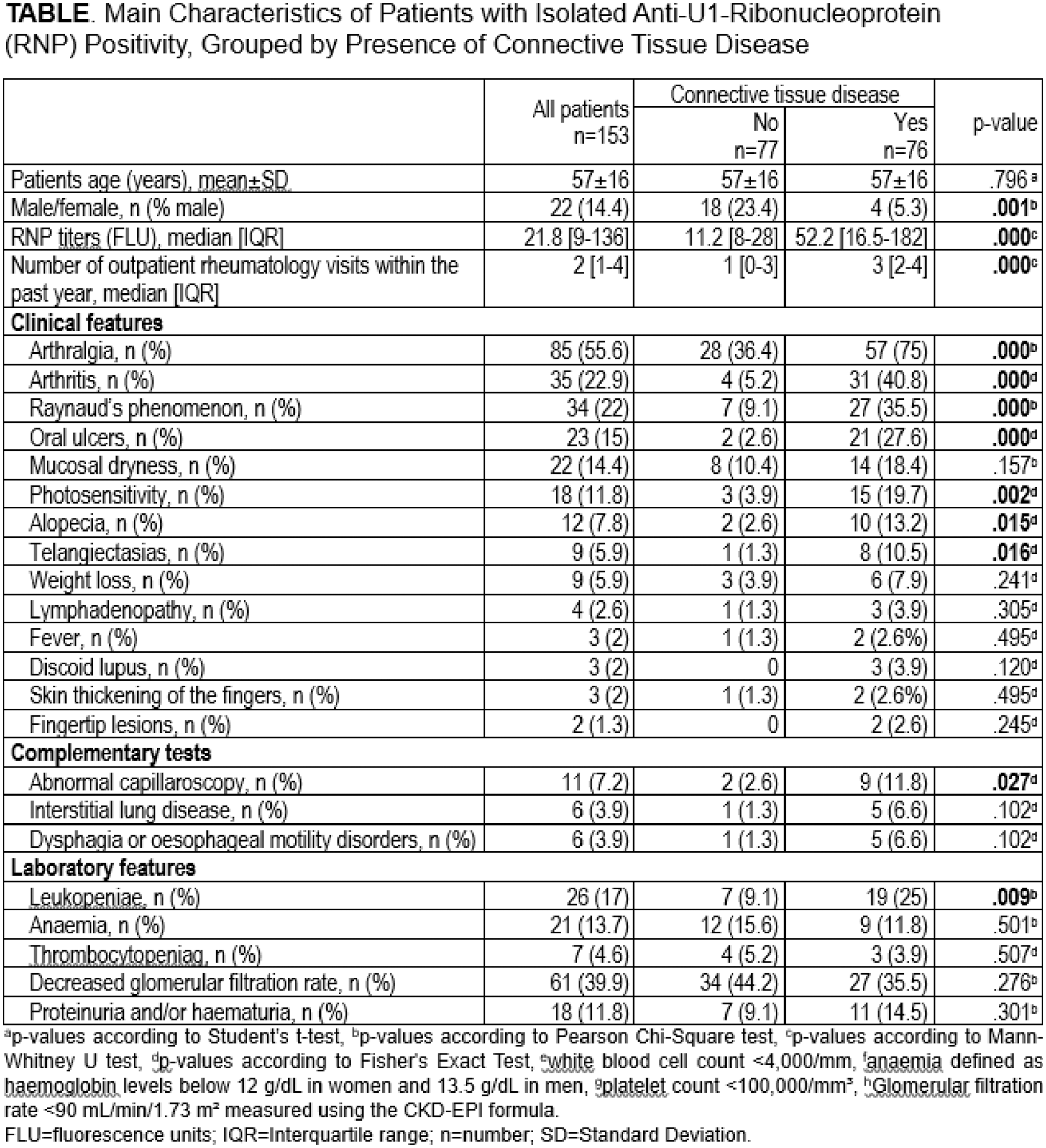
Results: We analysed 153 patients (86% female; mean age 57 ± 16 years) with isolated anti-RNP positivity. An underlying connective tissue disease (CTD) was identified in 76 patients (49.7%). Anti-RNP antibody FLU was significantly higher in patients with CTD (median 52.2 [16.5-182] vs 11.2 [8–28] FLU; p<0.001). A progressive increase in the prevalence of CTD was observed with rising anti-RNP FLU (p<0.001) Figure 1. Additionally, individuals with CTD had a greater number of outpatient rheumatology visits within the past year (median 3 [2–4]) compared to those without CTD (1 [0–3], p<0.001). The frequency of underlying CTD were SLE (47.4%), undifferentiated connective tissue disease (23.7%), rheumatoid arthritis (10.5%), MCTD (5.3%), and Rhupus syndrome (5.3%). Patients with underlying CTD clinically presented more frequently arthralgia (75% vs 36.4%, p<0.001), arthritis (40.8% vs 5.2%, p<0.001), Raynaud’s phenomenon (35.5% vs 9.1%, p<0.001), oral ulcers (27.6% vs 2.6%, p<0.001), photosensitivity (19.7% vs 3.9%, p=0.002), and alopecia (13.2% vs 2.6%, p=0.015) and telangiectasias (10.5% vs 1.3%, p=0.016). Leukopenia (25% vs 9.1%, p=0.009) and capillaroscopic abnormalities (11.8% vs 2.6%, p=0.027) were also more frequent in CTD patients. The clinical and laboratory features are summarized in Table 1.
Conclusion: Isolated anti-RNP antibodies detected by PMAT on Aptiva are strongly associated with underlying CTD, particularly in patients with higher antibody titres. Identifying key clinical features such as Raynaud’s phenomenon, arthritis, and leukopenia in patients with elevated anti-RNP titres may aid early diagnosis and targeted management of underlying CTD.
REFERENCES: [1] Ferrara CA, et al. ImmunoTargets Ther. 2023. PMID: 37525698.
[2] Riemekasten G, Humrich JY, Hiepe F. Dubois’ Lupus Erythematosus and Related Syndromes. Elsevier. 2018.
[3] Lorca-Arce D, et al. Heliyon. 2024. PMID: 38778929.
Acknowledgements: NIL.
Disclosure of Interests: Ligia Gabrie: None declared, Mayra Garcia: None declared, Hector Miguel Ulloa-Alvarado: None declared, Juan Irure-Ventura: None declared, Diana Prieto-Peña: None declared, Ricardo Blanco Abbvie, Pfizer, Roche, Bristol-Myers, Lilly, Janssen, and MSD, Abbvie, MSD, and Roche, Marcos López Hoyos Werfen.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (