

Background: Many areas of early psoriatic arthritis (PsA) remain poorly understood. Sex may play a role in patient phenotype in PsA. APACHE is a national multicentre cohort of very early (less than one year duration) peripheral joint PsA.
Objectives: To analyse the characteristics of patients with very early PsA, and to compare them according to sex.
Methods: APACHE is an ongoing multicentric national prospective cohort (NCT03768271); inclusions and follow-up are ongoing. Patients included have: 1/ peripheral arthritis that began within the last 12 months; 2/ a personal or family history of psoriasis; 3/ a diagnosis of PsA according to their rheumatologist and 4/ no previous or ongoing treatment with targeted therapies. At the inclusion visit, data collected include demographic data, disease activity (swollen and tender joint counts, DAPSA score, past or present entheseal pain, past or present dactylitis, extra-MSK features), and comorbidities. This interim analysis of the baseline visit of APACHE was descriptive for the whole population and differences between men and women (self-reported biological sex) were analysed (Wilcoxon, chi-2, Fisher; unadjusted analyses).
Results: Of the 193 patients included at the time of the analysis, 186 were analyzable. Mean age was 44 (SD 11) years, mean arthritis duration was 6 (SD 4) months, 91% had a personal history of psoriasis (for a mean duration of 14 years), and in terms of treatment, 41% were on non-steroidal anti-inflammatory drugs, 5% on glucocorticoids, and 38% on methotrexate at the time of inclusion. The sex ratio was balanced: 84 (45%) were women. The mean number of swollen and tender joints was 2.1 (SD 3.2) and 6.0 (SD 8.0) respectively; the first arthritis localization was mainly the hands (40%), wrists (27%) and knees (28%). CRP was normal for 51% patients. Entheseal pain (past or present: 39%) was more frequent than dactylitis (past or present: 27%). Disease activity according to DAPSA was moderate (mean 19, SD 14). Inflammatory bowel disease and uveitis concerned only 2 patients. Comorbidities were frequent: 27% patients were obese, 26% were smokers, and 49% had at least one cardio-vascular comorbidity/risk factor (CVCo). When comparing men and women, disease activity was similar in both groups (Figure 1). However, and as expected in that age group, CVCo were more frequent in men (at least one CVCo was present in 44% men versus 33% women, p=0.026).
Characteristics of men and women with very early PsA.
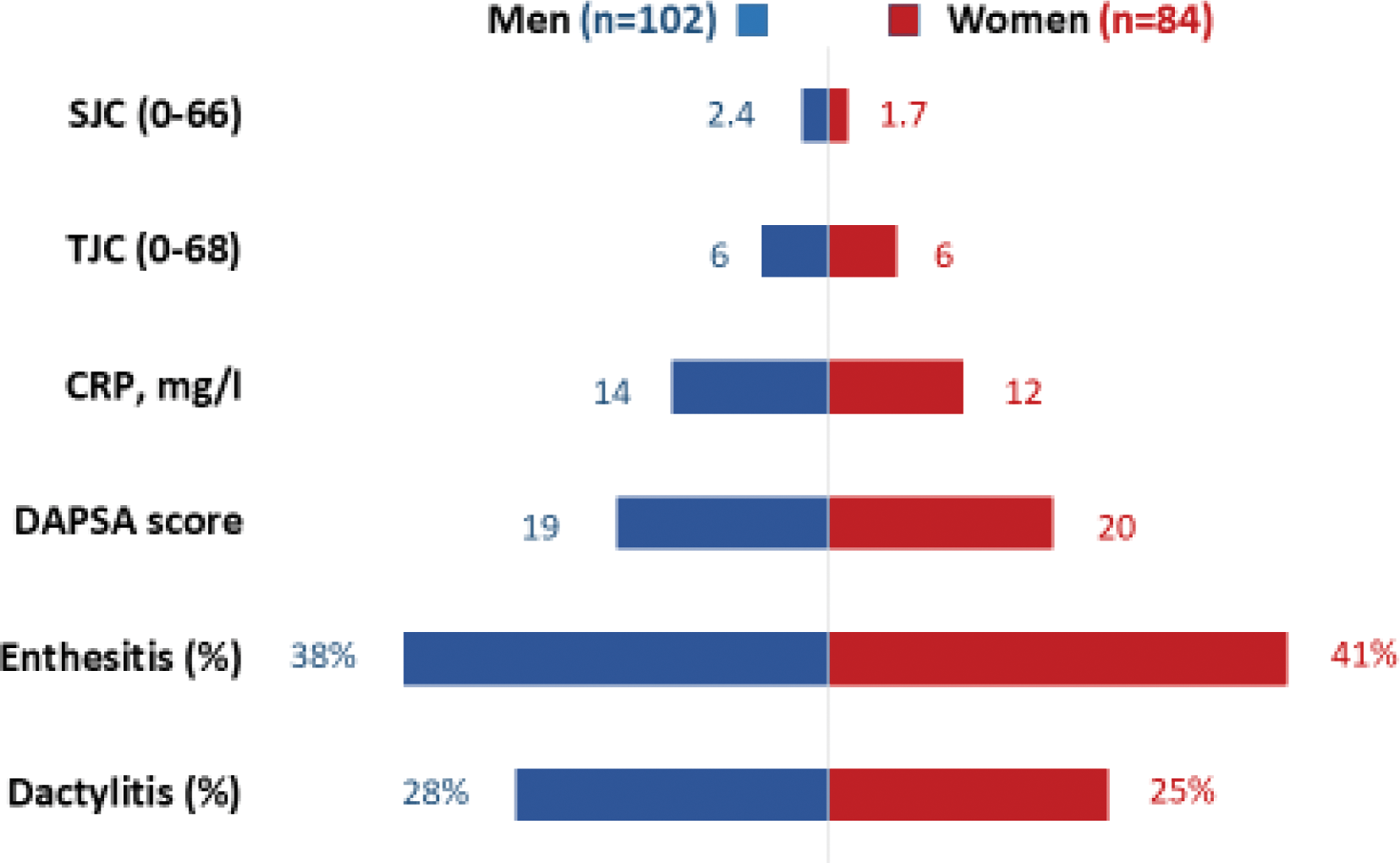
Conclusion: To our knowledge, this is the first description of very early (less than one year) peripheral PsA. The first joints concerned were mainly the hands and wrists; disease activity was mainly moderate, extra-joint features in particular entheseal pain were frequent, whereas extra-MSK features apart from psoriasis were rare. CVCo were frequent, in particular when compared to the French population at large, which has lower rates of CVCo than other countries (ref). At this early stage of the disease, the only clinical difference observed between men and women was the greater frequency of CVCo in men. The differences seen between men and women in later-stage PsA may be acquired over time; longer follow-up of the APACHE cohort will yield more information in this regard.
REFERENCES: [1] European Society of Cardiology Atlas, accessed Jan 6, 2025.
Acknowledgements: The APACHE cohort is conducted with Assistance Publique-Hôpitaux de Paris (AP-HP, Paris France) as the sponsor, under the umbrella and with the support of the French Society of Rheumatology. The APACHE cohort is run with the support of unrestricted grants from (in order of decreasing support): UCB, Abbvie, Lilly, Novartis, Janssen, Amgen, Galapagos, Viatris and Biogen.
Disclosure of Interests: Laure Gossec Consultant: AbbVie, AlfaSigma, Amgen, BMS, Celltrion, Janssen, Lilly, MSD, Novartis, Pfizer, Stada, UCB, Grants: AbbVie, Biogen, Lilly, Novartis, UCB, Valerie Devauchelle-Pensec: None declared, Pascal Richette: None declared, Arnaud Constantin: None declared, Francis Berenbaum: None declared, Cécile Gaujoux-Viala CGV reports serving as a consultant and on a speaker’s bureau for AbbVie, Amgen, Biogen, Biocon, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celltrion HealthCare, Chugai, Eli Lilly, Fresenius Kabi, Galapagos, Gilead Sciences, Inc., Janssen, Medac, Merck-Serono, Mylan, Nordic Pharma, Novartis, Pfizer, Sandoz, Sanofi, UCB and Viatrix, Philippe Goupille: None declared, Daniel Wendling: None declared, Gael Mouterde: None declared, Tristan Pascart: None declared, B Razat: None declared, Etienne Audureau: None declared, Sandrine Jousse-Joulin: None declared, Bernard Combe: None declared, Pascal Claudepierre .The APACHE cohort is run with the support of unrestricted grants from (in order of decreasing support): UCB, Abbvie, Lilly, Novartis, Janssen, Amgen, Galapagos, Viatris and Biogen.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (