

Background: Patients with autoimmune diseases (ADs) often exhibit systemic low-grade inflammation, commonly reflected in elevated C-reactive protein (CRP) levels. CRP, a plasma protein produced by the liver, is a crucial component of the innate immune system, playing a key role in recognizing pathogens and altered self-determinants [1]. Observational studies have consistently shown a significant association between CRP levels and the risk of Ads [2, 3]. However, the causal nature of this association remains unclear. While observational studies provide valuable insights, they are often subject to selection bias, residual confounding, and reverse causality, which complicate the interpretation of these associations. To address these limitations, Mendelian randomization (MR) has emerged as a promising approach. By leveraging genetic variants as instrumental variables, MR helps reduce confounding and reverse causality, providing stronger evidence for causal relationships between CRP levels and Ads [4].
Objectives: This study aimed to infer the causality between CRP and the risk of six ADs by means of the MR analysis.
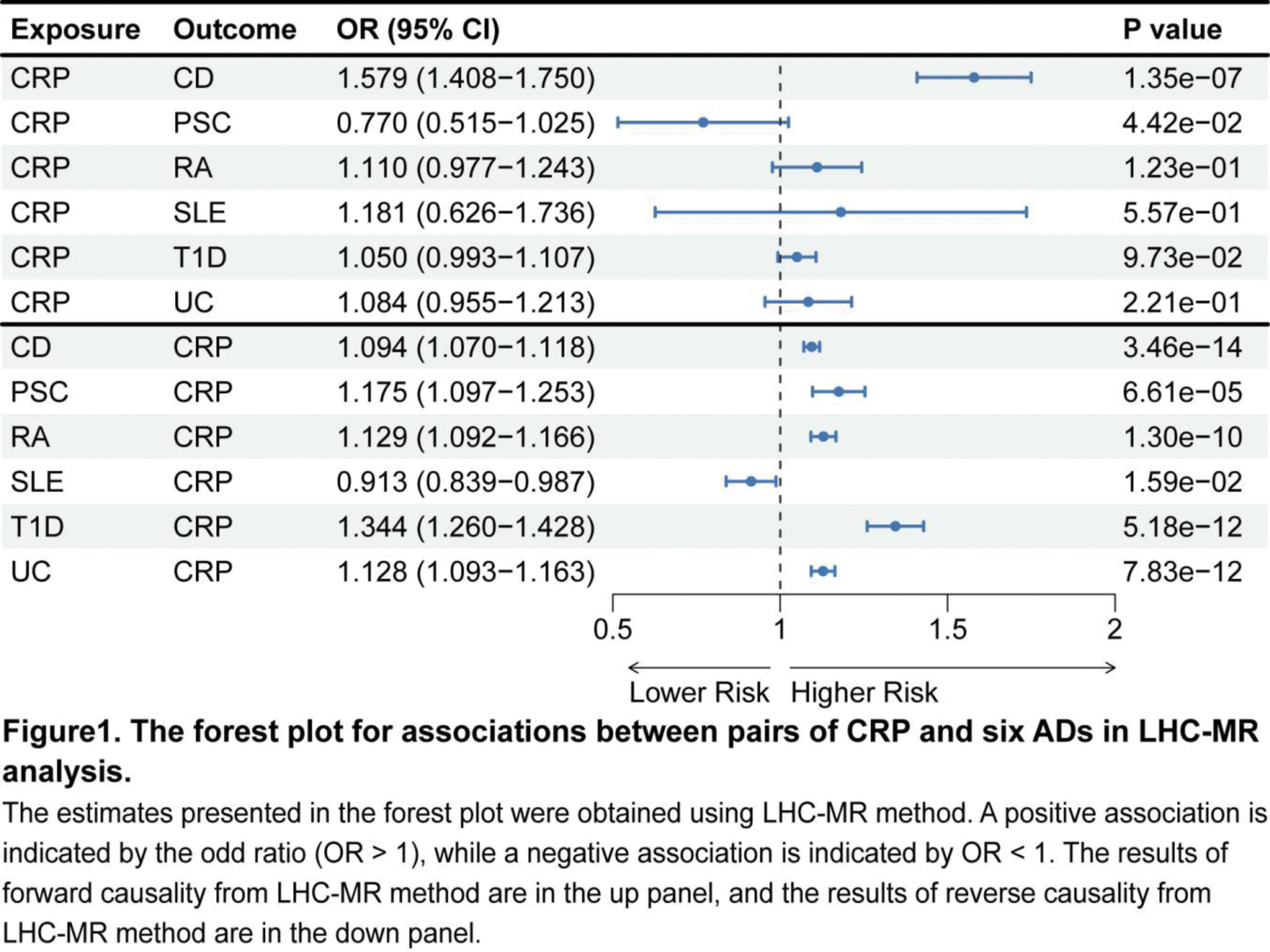
Methods: This study investigated the bidirectional effects between CRP and six major ADs: rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), type 1 diabetes (T1D), Crohn’s disease (CD), ulcerative colitis (UC), and primary sclerosing cholangitis (PSC). To achieve this, we utilized extensive GWAS summary data from individuals of European ancestry. The linkage disequilibrium heterogeneity causal Mendelian randomization (LHC-MR) [5] method was applied to assess bidirectional causality between CRP and these ADs. LHC-MR extends the standard two-sample MR approach by modeling potential heritable confounders that influence both the exposure and outcome. This method addresses some key limitations of traditional two-sample MR, such as sample overlap, under-utilization of genome-wide markers, and the challenge of accurately understanding exposure-outcome relationships. To ensure the robustness of our findings, we applied a Bonferroni correction to account for multiple comparisons. When the P-value in one direction was below the significance threshold (P = 4.17 × 10 -3 ) and the P-value in the opposite direction exceeded 0.05, it indicated a one-way causal relationship. In cases where the P-values in both directions exceeded the threshold, we concluded that a bidirectional causal relationship likely existed.
Results: The results of the study revealed a partial causal relationship between CRP levels and ADs. Specifically, we found that RA, T1D, and UC all led to increased CRP levels, with odds ratios (ORs) of 1.129 (95% CI, 1.092–1.166), 1.344 (95% CI, 1.260–1.428), and 1.128 (95% CI, 1.093–1.163), respectively. These findings align with previous studies that have demonstrated elevated CRP levels in ADs patients. For example, in randomized clinical trials (RCTs) assessing RA treatments, baseline CRP levels above 20 mg/L are commonly reported, reflecting chronic elevation in RA patients 3 . Additionally, Schalkwijk et al. observed higher plasma CRP concentrations in T1D patients without clinical macroangiopathy compared to control subjects, further supporting our findings [6]. We also observed a significant bidirectional causal relationship between CRP and CD. Specifically, elevated CRP levels contributed to an increased risk of CD, while the presence of CD also led to higher CRP levels in patients. However, the role of CRP in UC appears less consistent. Although a previous study indicated that higher CRP was an independent predictor of disease relapse in quiescent CD [7], a study in UC patients found no association between CRP and disease recurrence [8]. This discrepancy suggests that the relationship between CRP and inflammatory bowel diseases may vary depending on disease type. Notably, the LHC-MR analysis did not reveal any causal relationship between CRP levels and SLE or PSC.
Conclusion: Our results show an association between elevated CRP levels and susceptibility to RA, T1D, and UC, which helps provide new insights into the clinical research of CRP and these diseases.
REFERENCES: [1] Cooper J, Pastorello Y, Slevin M. A meta-analysis investigating the relationship between inflammation in autoimmune disease, elevated CRP, and the risk of dementia. Frontiers in immunology. 2023;14:1087571.
[2] Rezaieyazdi Z, Sahebari M, Hatef MR, et al. Is there any correlation between high sensitive CRP and disease activity in systemic lupus erythematosus? Lupus. 2011;20(14):1494-1500.
[3] Pope JE, Choy EH. C-reactive protein and implications in rheumatoid arthritis and associated comorbidities. Seminars in arthritis and rheumatism. 2021;51(1):219-229.
[4] Zhou A, Hyppönen E. Vitamin D deficiency and C-reactive protein: a bidirectional Mendelian randomization study. International journal of epidemiology. 2023;52(1):260-271.
[5] Darrous L, Mounier N, Kutalik Z. Simultaneous estimation of bi-directional causal effects and heritable confounding from GWAS summary statistics. Nature communications. 2021;12(1):7274.
[6] Schalkwijk CG, Poland DC, van Dijk W, et al. Plasma concentration of C-reactive protein is increased in type I diabetic patients without clinical macroangiopathy and correlates with markers of endothelial dysfunction: evidence for chronic inflammation. Diabetologia. 1999;42(3):351-357.
[7] Bitton A, Dobkin PL, Edwardes MD, et al. Predicting relapse in Crohn’s disease: a biopsychosocial model. Gut. 2008;57(10):1386-1392.
[8] Bitton A, Peppercorn MA, Antonioli DA, et al. Clinical, biological, and histologic parameters as predictors of relapse in ulcerative colitis. Gastroenterology. 2001;120(1):13-20.
Acknowledgements: This work was financially supported by the National innovation and EntrepreneurshipTraining Program for College Students, China (20240416).
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (