

Background: Anti-citrullinated peptide autoantibody seropositive rheumatoid arthritis (SPRA) and psoriatic arthritis (PsA) are relatively well-characterised disease phenotypes for which a growing number of rational therapies target defined pathways. Other forms of seronegative immune-mediated inflammatory arthritis (SIA) – including undifferentiated inflammatory arthritis and seronegative RA – are more heterogeneous in presentation, difficult to classify and lack rational treatment strategies. Immune checkpoint inhibitor-induced inflammatory arthritis (ICI-IA), which develops in ≥6% cancer patients treated with ICIs, shares many clinical characteristics with SIA and is similarly under-represented in interventional clinical trials.
Objectives: Identify common and unique disease mechanisms of SIA and ICI-IA with SPRA and PsA, as potential targetable disease pathways for patients poorly served by existing treatments.
Methods: Synovial tissues were obtained from patients who were naïve to glucocorticoids or disease modifying anti-rheumatic drugs across two recruitment sites. Human biological samples were sourced ethically, and their research use was in accord with the terms of the informed consents under a REC approved protocol. Single-cell RNA-Seq was performed using the 10X platform after enzymatic disaggregation [1] and live cell sorting. Paired paraffin-embedded tissues were haematoxylin and eosin (H+E)-stained and scored for synovitis severity [2]; Xenium spatial transcriptomics is currently underway for a subset of these samples.
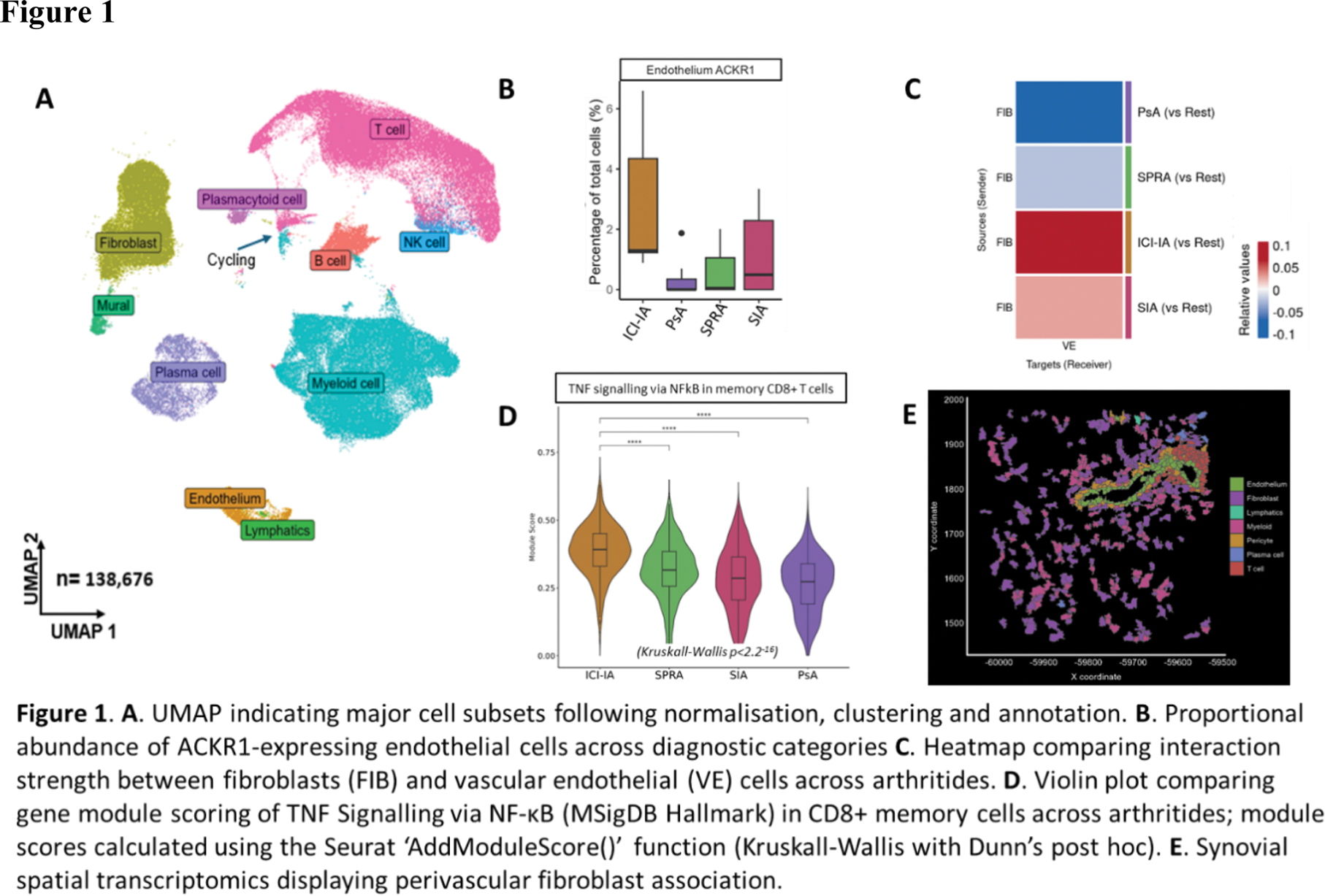
Results: Synovitis severity did not differ significantly between disease phenotypes (median Krenn score 5). 138,676 cells were sequenced from 36 individuals (11 SPRA, 7 PsA, 9 SIA and 9 ICI-IA) with major cell lineages clearly delineated following normalisation, clustering and annotation (Figure 1A). Considering relative cellular abundance, a higher proportion of ICI-IA tissues fell within a “fibroblast dominant” (as opposed to myeloid and/or T cell-dominant) cluster, compared with those from participants with “spontaneous” arthritis (45% versus 7%; p=0.024, Fisher’s Exact test). In addition, ICI-IA tissues exhibited a significant preponderance of the endothelial cell markers PODXL, KDR and ACKR1 compared with those of comparator groups (5% false discovery rate [3]; Figure 1B). Correspondingly, inferred contact [4] between fibroblasts and endothelial cells was significantly enhanced in ICI-IA compared to either SPRA or PsA, and a similar pattern was observed for SIA, albeit less marked (Figure 1C). Other predicted disease group-specific cellular interactions included dendritic cells (DC) with CD8+ T-cells in ICI-IA, and gene set enrichment analysis highlighted relatively enhanced TNF signalling in memory CD8+ T cells (BH-corrected p=5.21 × 10 -5 ), reflecting a pattern in the synovium more generally; indeed, module scoring suggested this significantly exceeded that observed in SPRA (p=3.02 -49 ; Figure 1D). Spatial corroboration of these and other putative interactions at a transcript level by Xenium analysis is ongoing (Figure 1E).
Conclusion: Our preliminary findings highlight aspects of synovial pathobiology in ICI-IA that are shared to varying degrees with spontaneous arthritis subtypes. These insights already provide pathobiological support to the current use of TNF inhibitors as a treatment for ICI-IA and could guide the development of targeted treatment strategies for both ICI-IA and SIA in the future.
REFERENCES: [1] Donlin LT et al (2018).
[2] Krenn V et al (2002).
[3] Mangiola S et al (2023).
[4] Jin S et al (2024).
[5] Korotgevich G et al (2021).
Acknowledgements: This work was supported by the Medical Research Council (MR/X02914X/1), GSK and the JGW Patterson Foundation (30015.088/29of20/PA/IXS). Infrastructural support was provided by Versus Arthritis (Research into inflammatory Arthritis CEntre; award ref. 22072) and the National Institute of Health and Care Research (NIHR) Newcastle and Birmingham Biomedical Research Centres. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Disclosure of Interests: Jason D. Turner: None declared, Anthony K. McLean: None declared, Amanda Thomson: None declared, Kristian Williams: None declared, Ioana Nicorescu: None declared, Luke Jones: None declared, George Merces: None declared, Ana Teodosio: None declared, Bethany Hunter: None declared, Jack McMurray: None declared, Sebastian Gilbert: None declared, Abbie Degnan: None declared, Dagmar Scheel-Toellner: None declared, Shashank Jariwala GSK shareholder, Current employee of GSK, Qin An GSK shareholder, Current employee of GSK, Ruth Plummer Recieved payment for delivery of educational talks or chairing educational meetings by AstraZeneca, Novartis, Bayer, MSD and BMS, Received honoraria for working as an IDMC member for Alligator Biosciences, GSK, Onxeo, SOTIO Biotech AG, and AstraZeneca. In the last 3 years received Honoraria for attending advisory boards from Pierre Faber, Bayer, Novartis, BMS, Ellipses, Immunocore, Genmab, Astex Therapeutics, MSD, Nerviano, AmLo, Incyte, Cybrexa Benevolent AI and Sanofi Aventis, Adam P. Croft: None declared, Lalit Pallan Recieved payment for speaking for Bristol Myers Squib, Merck, Recieved payment for consulting for Bristol Myers Squib, Iovance, Benjamin A. Fisher Recieved payment for speaking for Novartis, Servier, Novartis, BMS, Servier, Galapagos, Roche, UCB, Sanofi, Janssen, AZ, Otsuka, Amgen, Kiniksa, Recieved research funding from Janssen, Servier, Galapagos, Celgene, Novartis, Jonathan Coxhead: None declared, Rafiqul Hussain: None declared, Michal Magid-Slav GSK shareholder, Current employee of GSK, David McDonald: None declared, Stefan Siebert Receipt of departmental research grants from GSK, Andrew Filby: None declared, Gary Reynolds: None declared, Amy E Anderson: None declared, Catharien Hilkens: None declared, David F. Tough GSK shareholder, Current employee of GSK, Andrew Filer Consultancy for Johnson & Johnson and Sonoma, Institutional grant funding from BMS, Roche, UCB, Nascient, Mestag, GSK, Johnson & Johnson and Synact, Arthur Pratt Funding support from GSK for submitted abstract.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (