

Background: Idiopathic inflammatory myopathy (IIM) is a group of rare, systemic autoimmune rheumatic diseases, typically characterized by muscle weakness and other organ involvement. Efgartigimod is an IgG1 antibody Fc fragment that has been engineered for increased affinity to the neonatal Fc receptor (FcRn), and uniquely only contains that part of the IgG antibody that normally binds FcRn. Efgartigimod selectively reduces IgG by blocking FcRn-mediated IgG recycling without impacting antibody production, albumin levels, or other parts of the immune system. To date, there are very limited approved therapies for patients with IIM.
Objectives: To evaluate the safety and efficacy of subcutaneous (SC) efgartigimod (coformulated with recombinant human hyaluronidase PH20) compared with placebo in patients with IIM receiving standard of care (SOC) medications.
Methods: ALKIVIA (NCT05523167) is a seamless Phase 2/3, randomized, double-blinded, placebo-controlled, parallel-group, multicenter study in adult patients with active IIM. Included patients had subtypes of dermatomyositis, immune-mediated necrotizing myositis, polymyositis, or antisynthetase syndrome. Patients were randomized 1:1, stratified by IIM subtype and disease severity, to receive weekly efgartigimod PH20 SC or placebo, in addition to background SOC treatment for IIM. The primary endpoint was the 2016 ACR/EULAR Myositis Response Criteria’s Total Improvement Score (TIS) at Week 24. This composite endpoint comprises Physician’s Global Disease Activity (MDGA), Patient Global Disease Activity (PGA), manual muscle testing-8 (MMT8), Health Assessment Questionnaire Disability Index, muscle enzymes, and Extramuscular Global Disease Activity. Key secondary endpoints were time to reach TIS ≥20 and ≥40, and proportion of patients with TIS ≥20 and ≥40and changes in MMT8, PGA, and MDGA at Week 24.
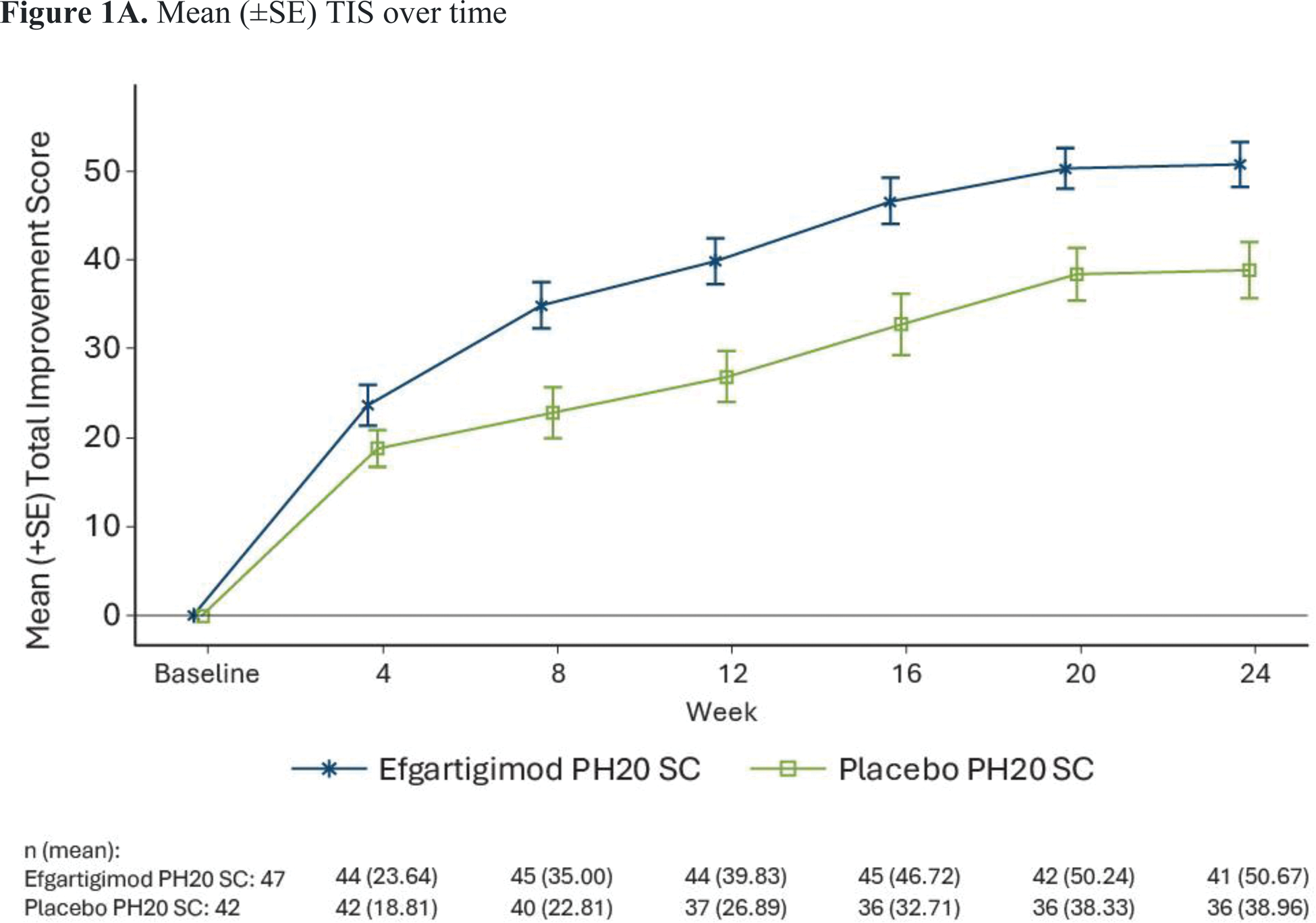
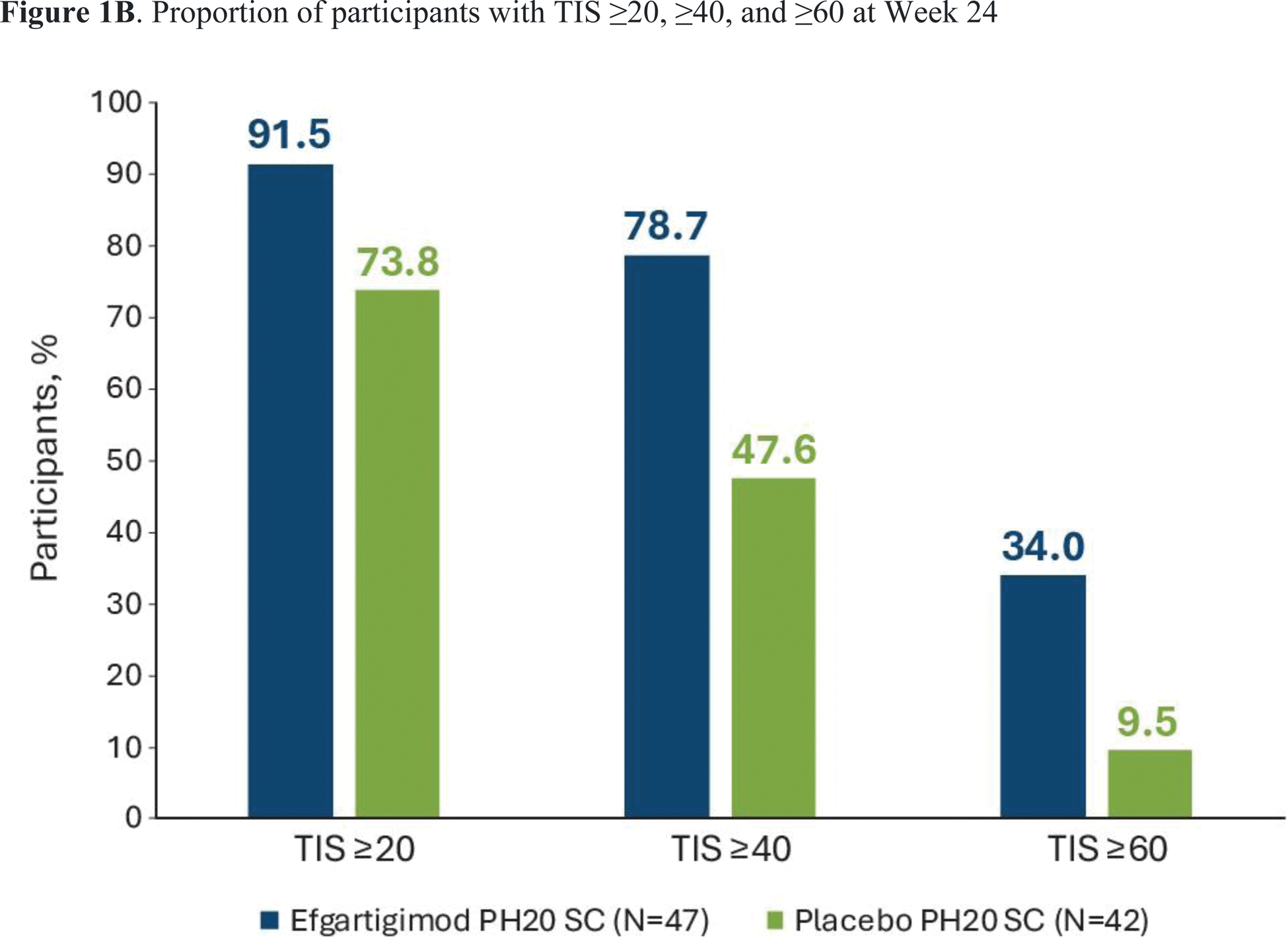
Results: The 24-week phase 2 study enrolled 89 participants with a mean age of 56.6 years and median time since diagnosis of 4.4 years; 76.4% were female and 71.8% were White. Participant demographics were generally comparable between treatment groups. At baseline, 76.4% were treated with disease-modifying anti-rheumatic drugs and 82.0% were treated with systemic corticosteroids. Least-squares mean TIS at Week 24 was statistically significantly higher in the efgartigimod PH20 SC arm than the placebo arm (50.45 vs 35.65, 2-sided P =0.0004) (Figure 1A). All key secondary endpoints were met, except for minimal clinical improvement (TIS ≥20) at week 24 (91.5% vs 73.8%). Median times to TIS ≥20 and ≥40 were significantly shorter with efgartigimod PH20 SC than with placebo (30 vs 71.5 days, P =0.0020; and 113 days vs not estimable, P =0.0293, respectively). A higher proportion of participants in the efgartigimod PH20 SC arm had a moderate (TIS ≥40) and major (TIS >60) clinical improvement at Week 24 compared with the placebo arm (78.7% vs 47.6% and 34.0% vs 9.5%, respectively) (Figure 1B). The proportion of patients experiencing ≥1 adverse event (AE) was similar in the 2 treatment groups (87.2% in the efgartigimod PH20 SC arm and 88.1% in the placebo arm; Table 1). Grade ≥3 AEs occurred in 14.9% of patients in the efgartigimod PH20 SC arm and 28.6% of patients in the placebo arm. Serious AEs occurred in 17% of participants in the efgartigimod PH20 SC arm and 21.4% in the placebo arm. The most common AEs in the efgartigimod PH20 SC arm were injection site erythema (23.4%), rash (17.0%), bruising (10.6%) or reaction (10.6%), and diarrhea (12.8%). There were 2 deaths in the efgartigimod PH20 SC arm (both unrelated to the study drug) and none in the placebo arm.
Conclusion: ALKIVIA is the first study of an FcRn inhibitor in IIM, establishing proof of concept. Efgartigimod PH20 SC led to significant improvement over placebo in TIS and key secondary endpoints with good safety and tolerability. The results demonstrate the mechanistic relevance of FcRn inhibition in IIM, indicating the potential pathogenicity of autoantibodies in IIM. These findings support further evaluation of efgartigimod PH20 SC in IIM in the ongoing phase 3 part of the study.
REFERENCES: NIL.
Mean (±SE) TIS over time
Proportion of participants with TIS ≥20, ≥40, and ≥60 at Week 24
Safety summary
| Efgartigimod PH20 SC
| Placebo PH20 SC
|
|||||
|---|---|---|---|---|---|---|
| n (%) | m | ER/100 PYFU | n (%) | m | ER/100 PYFU | |
| ≥1 AE | 41 (87.2) | 320 | 1474.1 | 37 (88.1) | 168 | 916.7 |
| ≥1 study drug-related AE | 21 (44.7) | 147 | 677.2 | 14 (33.3) | 53 | 289.2 |
| ≥1 SAE | 8 (17.0) | 11 | 50.7 | 9 (21.4) | 10 | 54.6 |
| ≥1 study drug-related SAE | 0 | 0 | 0 | 2 (4.8) | 2 | 10.9 |
| ≥1 Grade ≥3 AE | 7 (14.9) | 10 | 46.1 | 12 (28.6) | 13 | 70.9 |
| ≥1 corticosteroid-related AE | 12 (25.5) | 13 | 59.9 | 7 (16.7) | 11 | 60.0 |
| ≥1 IIM-related AE | 7 (14.9) | 10 | 46.1 | 3 (7.1) | 3 | 16.4 |
| ≥1 procedure-related AE | 15 (31.9) | 97 | 446.8 | 5 (11.9) | 23 | 125.5 |
| ≥1 AE leading to study drug discontinuation | 3 (6.4) | 3 | 13.8 | 4 (9.5) | 4 | 21.8 |
| ≥1 AE leading to study drug interruption | 9 (19.1) | 9 | 41.5 | 11 (26.2) | 18 | 98.2 |
| ≥1 AESI (infection) | 20 (42.6) | 24 | 110.6 | 20 (47.6) | 31 | 169.1 |
| ≥1 injection-related reaction | 13 (27.7) | 87 | 400.8 | 8 (19.0) | 27 | 147.3 |
| ≥1 injection-site reaction | 21 (44.7) | 155 | 714.0 | 9 (21.4) | 41 | 223.7 |
| ≥1 fatal AE | 2 (4.3) | 2 | 9.2 | 0 | 0 | 0 |
AE, adverse event; AESI, adverse event of special interest; ER, event rate; IIM, idiopathic inflammatory myositis; m, number of events; N, number of participants per treatment arm in the Safety Set; n(%), number and percentage of participants with adverse events per treatment arm; PYFU, participant years of follow-up; SAE, serious AE
Acknowledgements: Medical writing support was provided by Envision Pharma Group and was funded by argenx.
Disclosure of Interests: Hector Chinoy UCB, PTC therapeutics, AstraZeneca, Pfizer, Sebastian Rodriguez-Garcia Consultant for argenx, Agna Neto Consultant for argenx, Despoina Papadopoulou Employee of argenx, Ben Van Baelen Consultant for argenx, Paul Duncombe Consultant for argenx, Leentje De Ceuninck Employee of argenx, Bas van der Woning Employee of argenx, Rohit Aggarwal Alexion, ANI Pharmaceuticals, argenx, Artasome, AstraZeneca, Boehringer Ingelheim, Bristol Myers-Squibb, Cabaletta Bio, Capella Bioscience, Capstanx, Corbus, CSL Behring, EMD Serono, Galapagos, Horizon Therapeutics, I-Cell, Immunovant, Janssen, Kezar, Kyverna, Lilly, Manta Medicines, Novartis, Nuvig Therapeutics, Nkarta, Octapharma, Pfizer, PRoviant, Teva, Tourmaline Bio, Verismo Therapeutics, Boehringer Ingelheim, Bristol Myers-Squibb, EMD Serono, Janssen, Pfizer, Proviant.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (