

Background: As vasculopathy plays a pivotal role in the pathogenesis of systemic sclerosis (SSc), vasoactive vasodilating drugs (VVDs) represent a therapeutic option addressing the consequences of the disease on both peripheral and pulmonary vessels. Although most VVDs exert anti-fibrotic effects in pre-clinical studies, randomized controlled trials assessing the efficacy of VVDs in improving pulmonary function tests (PFTs) and preventing progression of SSc-associated interstitial lung disease (ILD) have shown mixed results.
Objectives: To assess the impact of VVDs on functional progression and all-cause mortality in SSc-ILD.
Methods: We performed a post-hoc analysis of prospectively collected data from the EUSTAR database, including SSc patients with ILD on high resolution computed tomography (HRCT), with follow-up information after ILD diagnosis and available treatment data. Patients with pulmonary hypertension on right heart catheterization were excluded. Treatment with VVDs included the categories of endothelin-receptor antagonists (ERA), phosphodiesterase-5 inhibitors (PDE5i) and prostanoids, administered for at least 3 months during the observation period. Three different outcomes for functional progression were defined within 12±3 months intervals: Progression A as a decline of forced vital capacity (FVC) ≥10%, or a decline in FVC of 5-9% combined with a decrease in diffusing capacity for carbon monoxide (DLCO) ≥15%; Progression B as a decline in FVC ≥5% or a decline in DLCO ≥10%, and Progression C as worsening of NYHA functional class. Generalized estimated equation mixed models, to account for multiple visits per patient, were performed separately for each outcome. A Cox-regression model with time-dependent variables was established for the survival analysis. Interaction terms were computed between VVDs and vascular parameters (digital ulcers – DU, DLCO). All models were adjusted for known risk factors for ILD progression or mortality respectively, including age, ethnicity, smoking status, antibody positivity, skin subset and exposure to immunosuppressants.
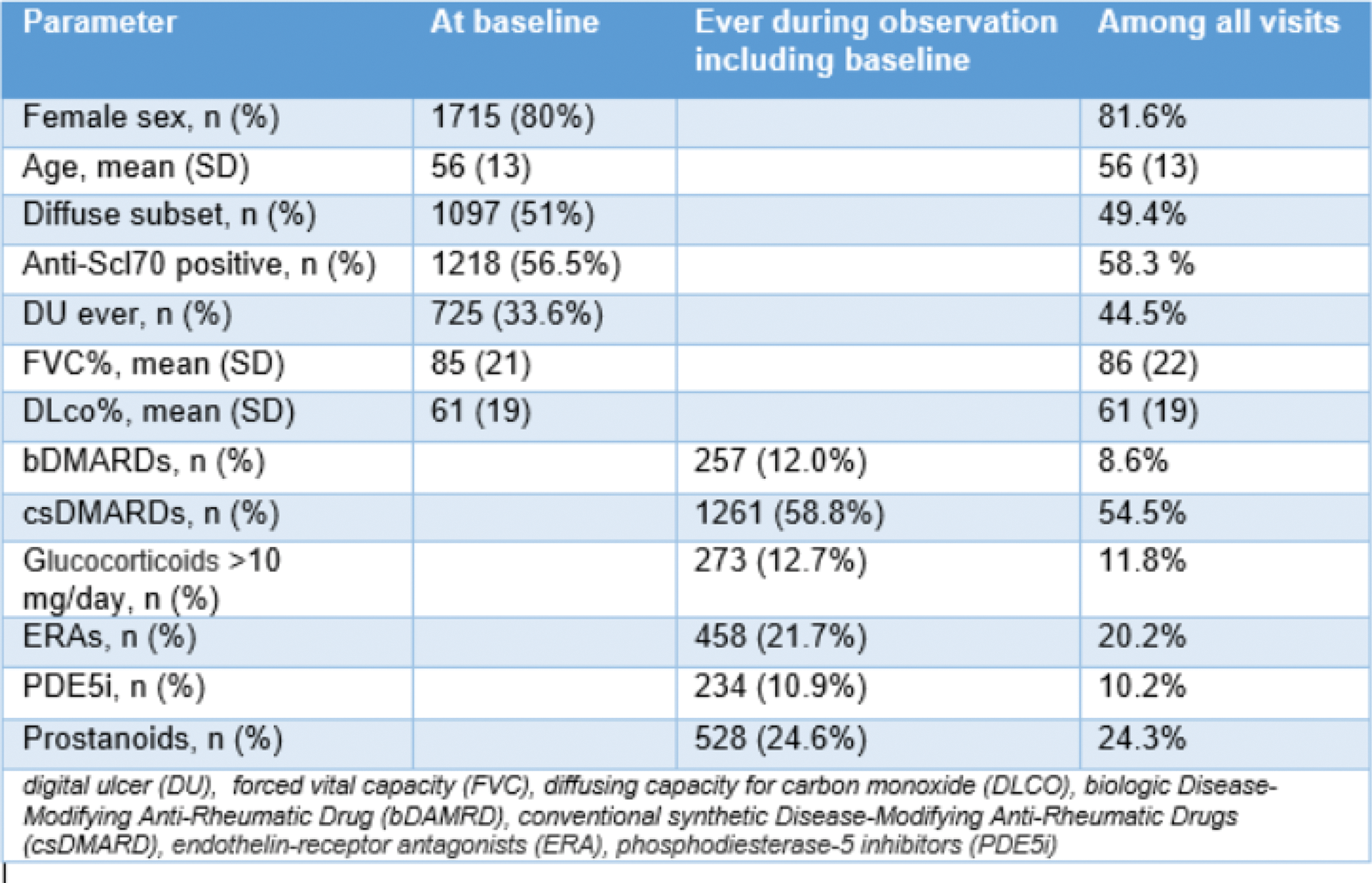
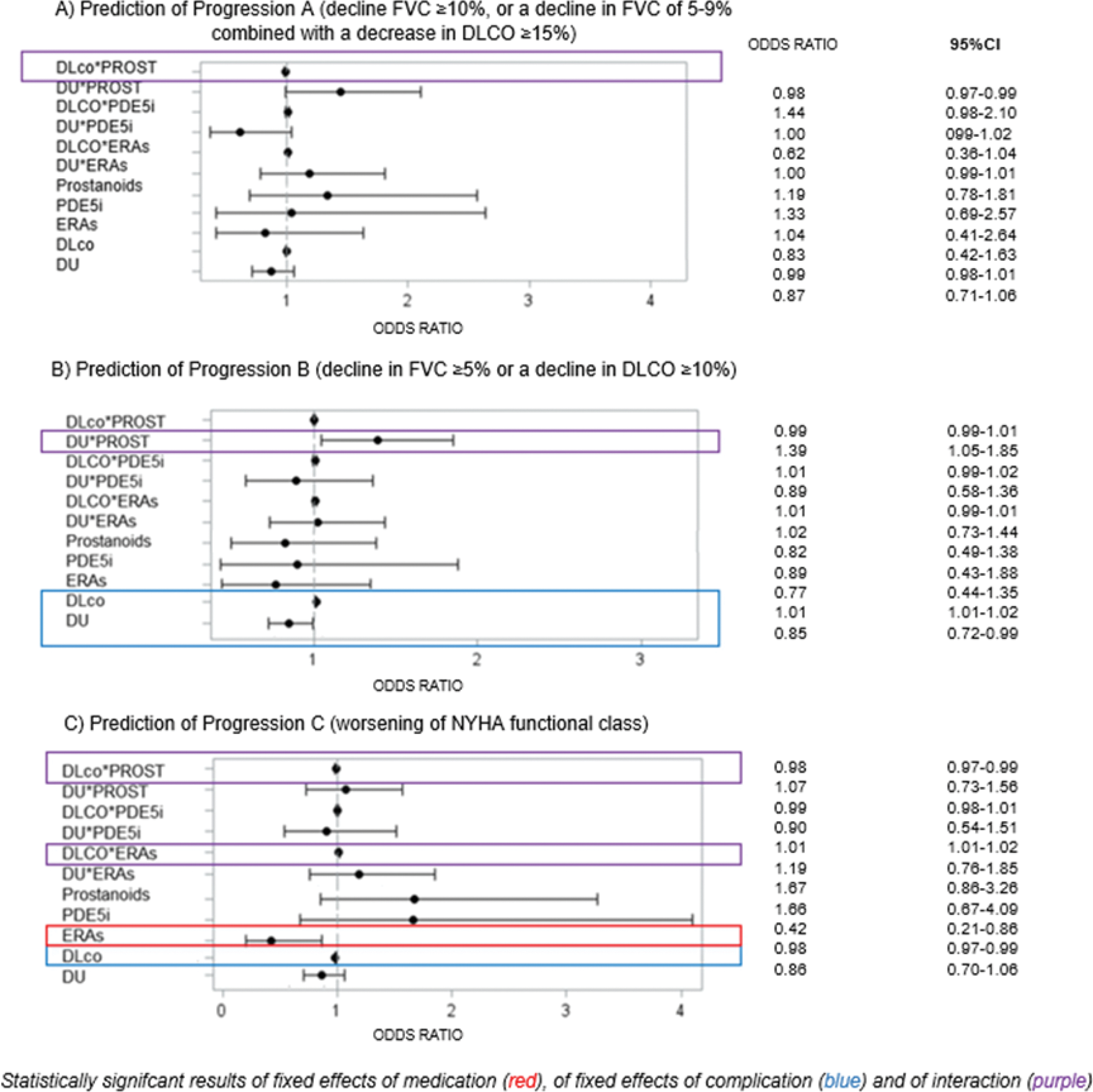
Results: Among 2156 patients with 5996 yearly visits, 80% were women, 51% had diffuse skin involvement and 56.5% were Scl-70 positive (additional information are presented in Table 1). Exposure to VVDs ranged from 10 to 25% of the visits, with prostanoids and ERAs being the most frequent categories. Progression A, B, C were recorded in 19.5% (933/4790), 42.7% (2172/5086) and 18.3% (1007/5499) of visits, respectively. When predicting progression A, prostanoids interacted positively with DLCO (OR 0.99 95% CI 0.98-1.01) and with digital ulcer (DU) (OR 0.87, 95% CI 0.71-1.06), both being significantly associated with the outcome – Figure 1A. The latter translated into a significant protective association of prostanoids against progression A in patients without DU, compared to patients in the same group not receiving these medications (OR 0.61, 95% CI 0.46-0.83). Similar results were held for the association of exposure to prostanoids and progression B – Figure 1B. In patients without DU, our analysis showed a protective association for the exposure to prostanoids against progression B, compared to unexposed patients (OR 0.76, 95% CI 0.60-0.94). For progression C, we observed that exposure to ERAs was significantly protective against worsening of NYHA class (OR 0.42, 95% CI 0.21-0.86), independently from the presence of DU. Interestingly, DLCO interacted differently with VVDs categories in this analysis: as a risk factor when combined with ERAs, while as a protective factor when interacting with prostanoids – Figure 1C. Over a median follow-up of 5.6 (2.5-9.8) years, 562/3599 (15.6%) SSc-ILD patients died. In the survival analysis, exposure to VVDs did not show any significant, independent impact on mortality.
Conclusion: Exposure to prostanoids is associated with lower risk of progression of ILD in patients with mild vasculopathy (no DU, higher DLCO). Conversely, exposure to ERAs is associated with lower risk of symptoms worsening, which seems more prominent in patients with more severe vasculopathy (lower DLCO). Given our positive preliminary results on short-term progression, but the lack of any independent impact on mortality, further studies are needed to confirm the beneficial effects of VVDs on SSc-ILD.
Table 1.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Adela-Cristina Sarbu: None declared, Liubov Petelytska Swiss National Research Foundation/Scholars at risk, Lorenzo Tofani: None declared, Gianluca Moroncini: None declared, Alexandra Balbir-Gurman: None declared, Elisabetta Zanatta: None declared, Jörg Henes: None declared, Paolo Airò Bohringer Ingelheim, Novartis, Roche, Bristol Myers Squibb, Eli Lilly, Amgen, CSL Bering, janssen, Marco Matucci-Cerinic Actelion Janssen Inventiva Bayer Biogen Boehringer CSL Behring Corbus Galapagos Mitsubishi Samsung Regeneron Acceleron MSD Chemomab Lilly Pfizer Roche, Actelion Astra Zeneca Janssen Inventiva Bayer Biogen Boehringer CSL Behring Corbus Argenx Galapagos Mitsubishi Samsung Regeneron Acceleron MSD Chemomab Lilly Pfizer Roche, Ana Maria Gheorghiu Boehringer Ingelheim, Foundation for Research in Rheumatology (FOREUM), Antonella Marcoccia: None declared, Branimir Anic: None declared, Jelena Colic: None declared, Daniel Furst CME only, Amgen, Novartis, Pfizer, Horizon, Emerald, Actelion, Amgen, BI, Novartis, Pfizer, Emerald, Roche/Genentech, Horizon, Prometheus, GSK, Astra-Zeneca, BI, Julia Spierings: None declared, Britta Maurer Boehringer Ingelheim, GaxoSmithKline, Novartis, Otsuka, and Merck Sharpe & Dohme;, mir-29 patent for the treatment of systemic sclerosis (US8247389, EP2331143), Novartis, Boehringer Ingelheim, Janssen-Cilag, and GaxoSmithKline, AbbVie, Protagen, and Novartis Biomedical Research financial support for congresses (ie, for registration fees and travel) from Medtalk, Pfizer, Roche, Actelion, Mepha, and Merck Sharpe & Dohme, Anna-Maria Hoffmann-Vold Boehringer Ingelheim, Janssen, Medscape, Merck Sharp & Dohme, Novartis and Roche, AbbVie, ARXX, Boehringer Ingelheim, Bristol Myers Squibb, Genentech, Janssen, Medscape, Merck Sharp & Dohme, Pliant Therapeutics, Roche and Werfen, Boehringer Ingelheim, Janssen, Oliver Distler 4P-Pharma, Abbvie, Acceleron, Acepodia Biotech, Aera, Alcimed, Altavant, Amgen, AnaMar, Anaveon AG, Argenx, AstraZeneca, Blade, Bayer, Boehringer Ingelheim, Calluna (Arxx), Cantargia AB, Catalyze Capital, Corbus, CSL Behring, Galderma, Galapagos, Glenmark, Gossamer, Horizon, Janssen, Kymera, Lupin, Medscape, MSD Merck, Miltenyi Biotec, Mitsubishi Tanabe, Nkarta Inc., Novartis, Orion, Pilan, Prometheus, Quell, Redxpharma, Roivant, EMD Serono, Topadur and UCB, CITUS AG, Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143), 4P-Pharma, Abbvie, Acceleron, Acepodia Biotech, Aera, Alcimed, Altavant, Amgen, AnaMar, Anaveon AG, Argenx, AstraZeneca, Blade, Bayer, Boehringer Ingelheim, Calluna (Arxx), Cantargia AB, Catalyze Capital, Corbus, CSL Behring, Galderma, Galapagos, Glenmark, Gossamer, Horizon, Janssen, Kymera, Lupin, Medscape, MSD Merck, Miltenyi Biotec, Mitsubishi Tanabe, Nkarta Inc., Novartis, Orion, Pilan, Prometheus, Quell, Redxpharma, Roivant, EMD Serono, Topadur and UCB, BI, Kymera, Mitsubishi Tanabe, UCB, Dr. Cosimo Bruni Boehringer Ingelheim, Novartis Foundation for medical-biological research, EMDO Foundation, Iten-Kohaut Foundation. Educational grants from Wellcome Trust. Congress support from Boehringer-Ingelheim, Hartmann-Muller Foundation.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (