

Background: Gout flares are temporally associated with cardiovascular events. Treating gout to target (T2T) serum urate (SU) level prevents gout flares. Whether such treatment can prevent cardiovascular events is unknown.
Objectives: To evaluate the effect of achieving SU <360 µmol/l within 12 months of first prescription of urate-lowering therapy (ULT) on the 5-year risk of major adverse cardiovascular events (MACE) among patients with gout.
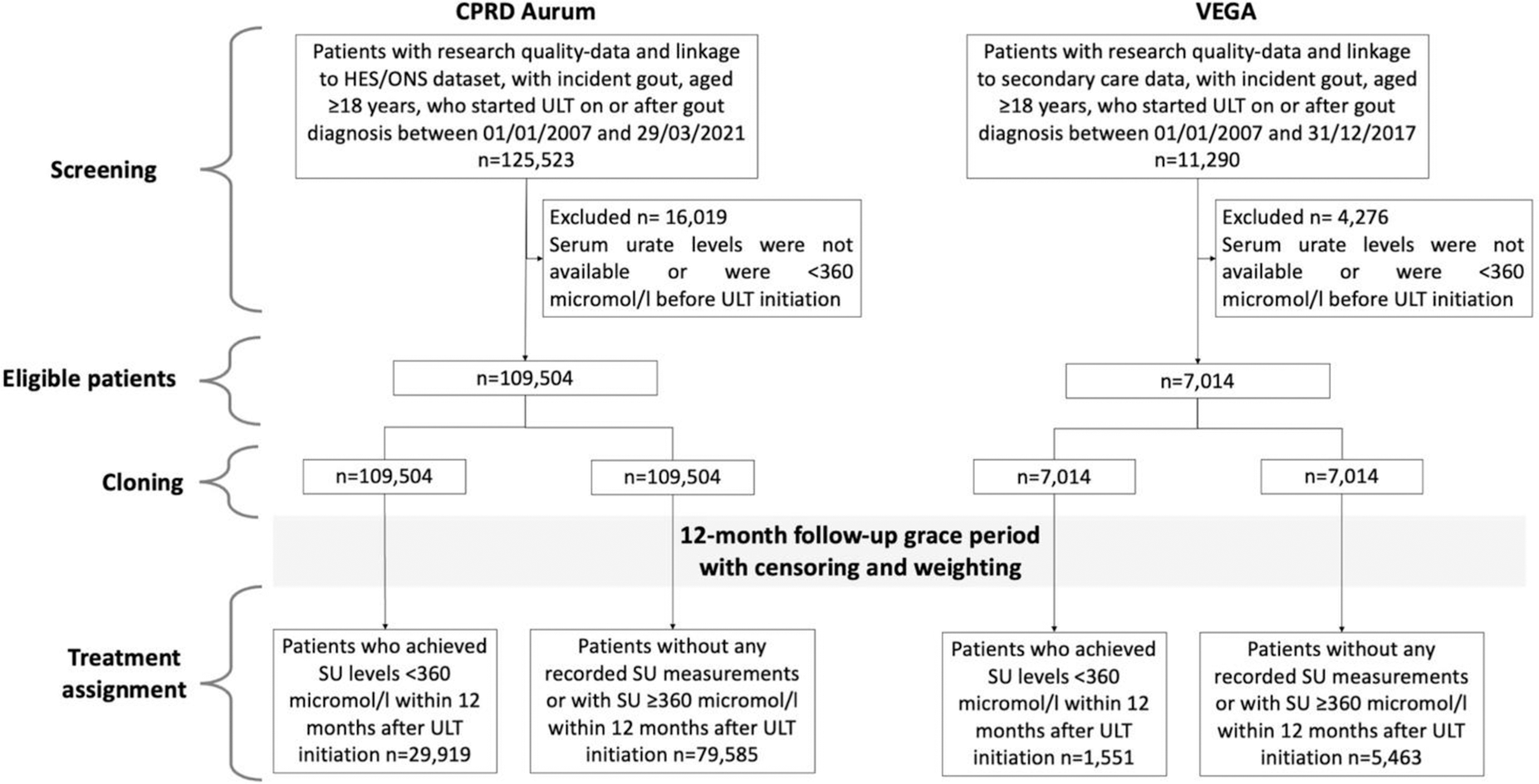
Methods: English and Swedish primary-care data linked to hospitalization and mortality records were obtained from Clinical Practice Research Datalink (CPRD) Aurum (01/01/2007 - 29/03/2021) and Western Sweden Regional Healthcare Database (VEGA) (01/01/2007 - 31/12/2017). We included people prescribed ULT on or after first recorded gout diagnosis with the latest pre-treatment SU >360 µmol/l. We assigned patients to the T2T-ULT arm if they had a recorded SU measurement <360 µmol/l within 12 months after ULT initiation. We assigned patients to the fire-and-forget arm if they had not any recorded SU measurements within 12 months after ULT initiation or they had persistent SU levels >360 micromol in the 12 months after ULT initiation. Patients were followed up from ULT initiation to up to 5 years. The first MACE after cohort entry (i.e., non-fatal myocardial infarction, non-fatal stroke, or cardiovascular death) was the primary outcome. Outcomes were ascertained using primary care, secondary care and mortality records. Secondary outcomes were first-ever MACE (i.e., excluding patients with previous MACEs before cohort entry), MACE requiring hospitalisation or leading to death (i.e., disregarding MACEs ascertained in primary care only), myocardial infarction, and stroke. We evaluated the number of gout flares as positive control outcome and acute bronchitis, cataract, and appendicitis as negative control outcomes. 40 baseline covariates were ascertained at cohort entry. They included demographic and socioeconomic variables, traditional cardiovascular risk factors, gout-related variables, cardioactive and gout-specific medications. We performed an emulated target trial using a cloning, censoring, and weighting approach to to minimise immortal time bias, lead time bias, and survivor bias related to the lack of alignment between the start of follow-up and the assignment of the treatment strategy [1]. We estimated the 5-year overall survival and survival difference using a non-parametric Kaplan-Meier estimator weighted for the inverse conditional probability of being censored (conditioned on baseline covariates) [2]. The 95% confidence intervals (95%CI) were obtained using non-parametric bootstrap with 100 replicates. We calculated hazard ratios (HR) and rate ratios and their 95%CIs using robust standard error estimators. Results from English and Swedish data were meta-analysed using a random effect model.
Results: We included data from 116,518 patients, 109,504 and 7,014 from CPRD Aurum and VEGA respectively (Figure 1). Patients in CPRD Aurum were younger than those in VEGA (mean (SD) age 62.9 (15.2) vs. 70.0 (14.7) years), less often female (24,358 (22.2%) vs. 1,985 (28.3%)), had longer duration of gout since the diagnosis (mean (SD) 2.5 (3.6) vs. 1.1 (1.8) years) and lower pre-treatment serum urate level (mean (SD) 512.4 (83.3) vs. 547.0 (106.8)) mol/l). In both populations, baseline covariates were well-balanced after weighting (standardised mean difference ≤0.10). 15,081 (13.77%) and 1,120 (15.97%) patients had a MACE over a mean (SD) follow-up time of 3.45 (1.71) and 2.62 (1.75) years in CPRD Aurum and VEGA respectively. The 5-year weighted survival rates in T2T-ULT and fire-and-forget ULT arms were 89.43% (95%CI: 88.97-89.89) and 88.03% (95%CI: 87.73-88.33) (survival difference: 1.40%, 95%CI: 0.85-1.95, hazard ratio: 0.88, 95%CI: 0.86-0.89) in the CPRD dataset and 76.82% (95%CI: 74.03-79.61) and 75.50% (95%CI: 73.99-77.01) (survival difference: 1.32%, 95%CI: -1.86 to 4.50, hazard ratio: 0.94, 95%CI: 0.84-1.04) in the VEGA dataset. After meta-analysis, patients who achieved SU<360 mol/L within 12 months had a higher weighted 5-year MACE-free overall survival (pooled survival difference (95% CI) 1.40% (0.86-1.94)) and lower risk of MACE compared with those who did not (pooled HR (95% CI) 0.89 (0.84-0.94)). Findings were similar in analyses exploring different MACE definitions, when follow-up was censored on ULT discontinuation, when patients without available SU during the first year of follow-up were excluded, and when each component of MACE was considered separately. There was an interaction between age and ULT strategies, as the effect size in people aged>65 years was significantly greater than those aged ≤65 years, respectively (p interaction 0.02). A significantly lower number of flares was recorded in those who achieved SU<360 mol/L (pooled weighted 5-year incidence rate ratio: 0.95, 95%CI: 0.92-0.99). We did not observe significant differences in the 5-year weighted survival rates and weighted HRs for negative control outcomes.
Patient selection flow chart
Random-effect meta-analysis of the weighted 5-year MACE-free survival difference (SD) and weighted HR comparing the effect of T2T-ULT versus fire-and-forget ULT
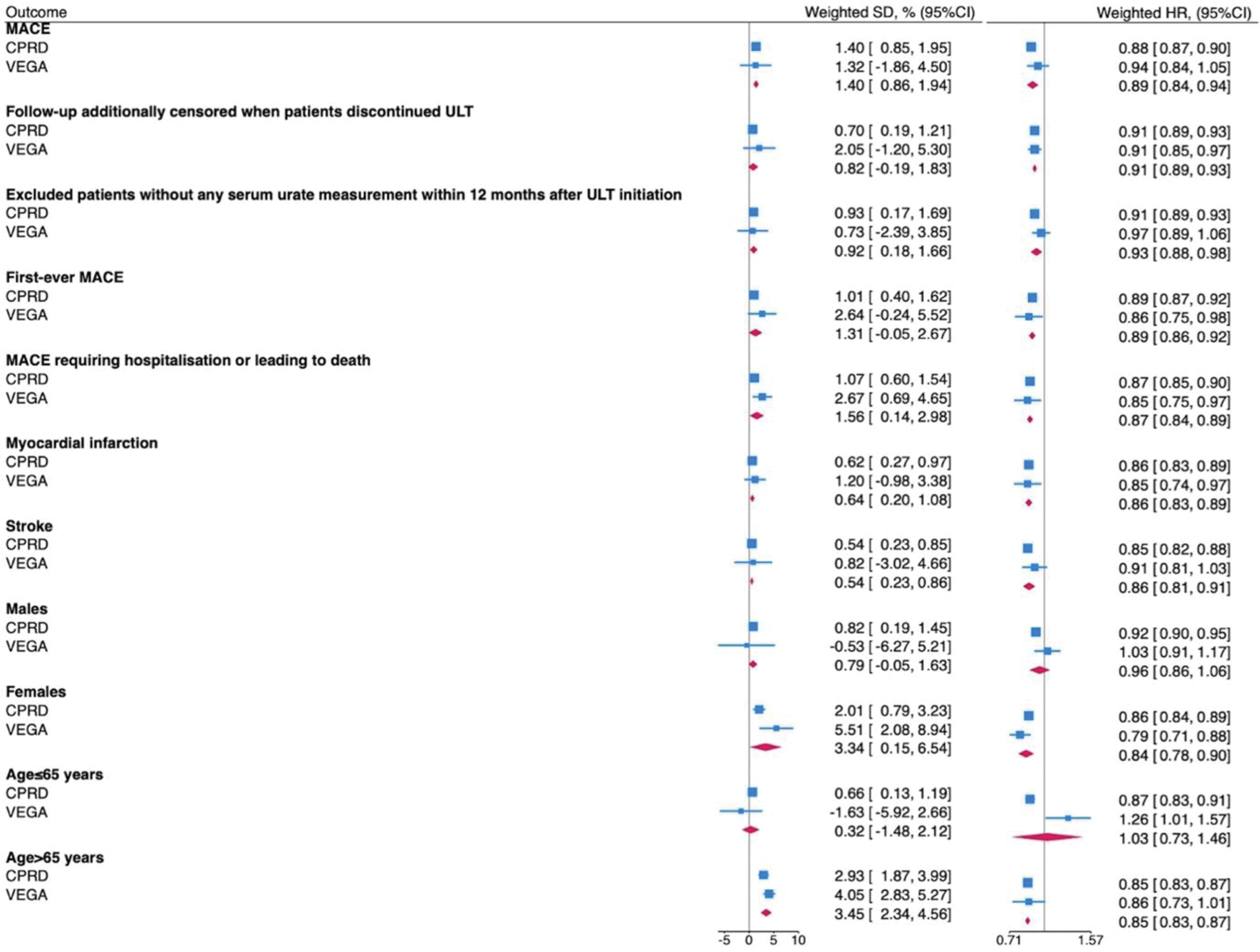
Conclusion: T2T-ULT significantly lowered the risk of MACE by a mild magnitude in two independent European datasets without evidence of residual confounding.
REFERENCES: [1] Hernán MA, et al. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol . 2016;79:70-5.
[2] Maringe C, et al. Reflection on modern methods: trial emulation in the presence of immortal-time bias. Assessing the benefit of major surgery for elderly lung cancer patients using observational data. Int J Epidemiol . 2020;49:1719-29.
Acknowledgements: This work was supported by a research grant from FOREUM Foundation for Research in Rheumatology.
Disclosure of Interests: Edoardo Cipolletta Horizon Therapeutics, Tatiana Zverkova Sandstrom: None declared, Davide Rozza: None declared, Clemence Leyrat: None declared, Georgina Nakafero: None declared, Panagiota Drivelegka Horizon Therapeutics, Anthony Avery: None declared, Mamas Mamas: None declared, Laila Tata: None declared, Mats Dehlin: None declared, Abhishek Abhishek Limbic (consulting), UpToDate (Royalty), Cadilla Pharmaceuticals and SOBI (Lecture fees).
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (