

Background: Lupus nephritis (LN) is the most common, severe, organ-threatening manifestation of systemic lupus erythematosus (SLE). Improvement or stabilisation of kidney function and improvement of proteinuria, key goals in clinical practice, are commonly included in the composite primary endpoint definitions of complete response in recent LN clinical studies. Nevertheless, as there is no standardised definition of complete response, studies utilise different surrogates of kidney function and set different cut-off values for each of the individual components of the complete response definition. The Phase III REGENCY study (NCT04221477) demonstrated superiority of obinutuzumab over placebo in achieving complete renal response (CRR) at Week 76 when added to standard therapy in patients with active LN.
Objectives: To evaluate the effect of obinutuzumab plus standard therapy (mycophenolate mofetil plus glucocorticoids) versus placebo and standard therapy across different renal response definitions used in recent pivotal studies.
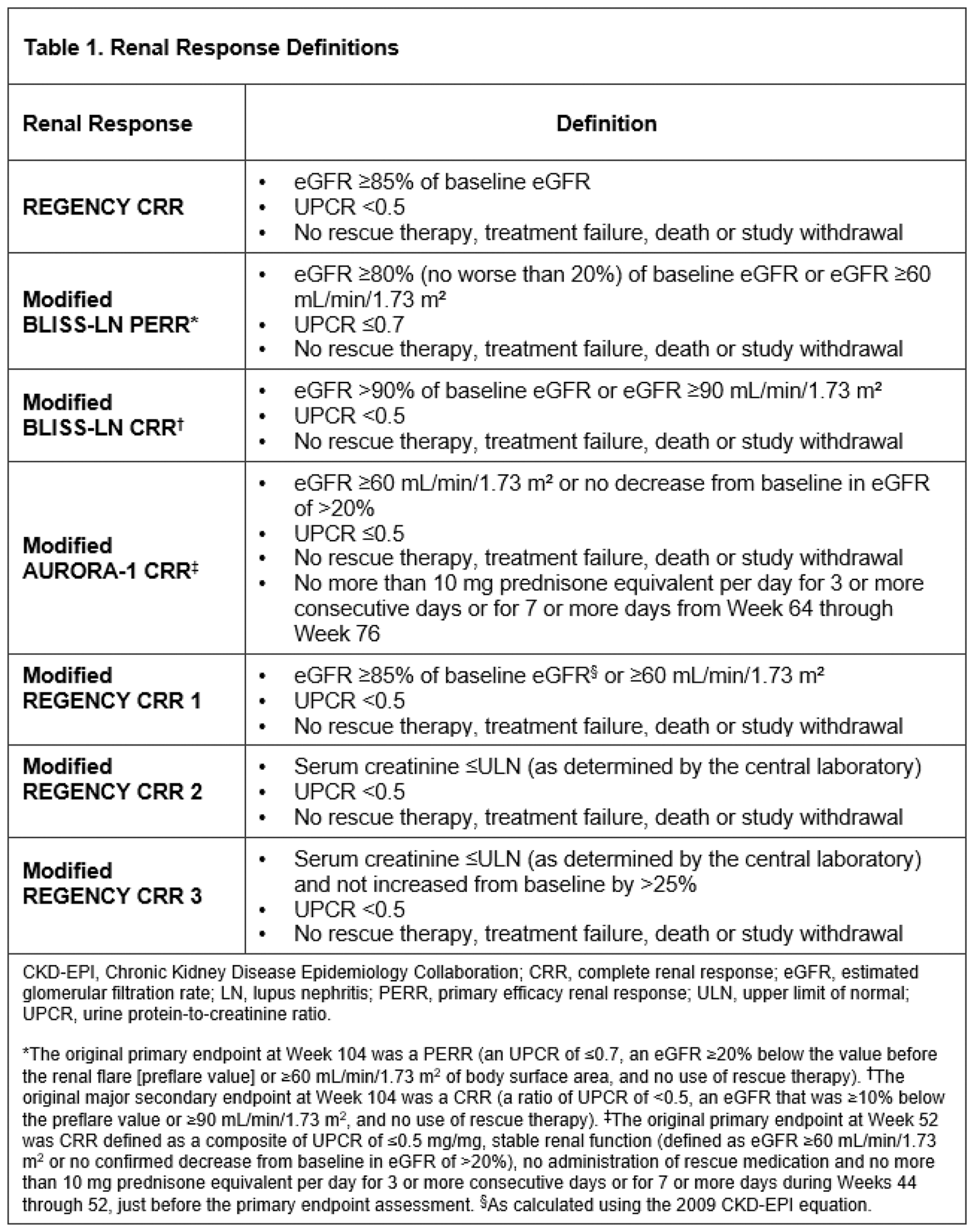
Methods: REGENCY primary and other pre-specified renal response endpoints, as well as BLISS-LN and AURORA-1 primary or secondary renal endpoint definitions, were used for this post hoc analysis using the REGENCY dataset at Week 76. Endpoint measures were defined according to those listed in Table 1. Analyses were performed in the intention-to-treat population. The proportions of patients achieving CRR in the two groups were compared using a Cochran–Mantel–Haenszel test with region and race as stratification factors with multiple imputation for missing data. All patients met the American College of Rheumatology classification criteria for SLE and had biopsy-proven active LN.
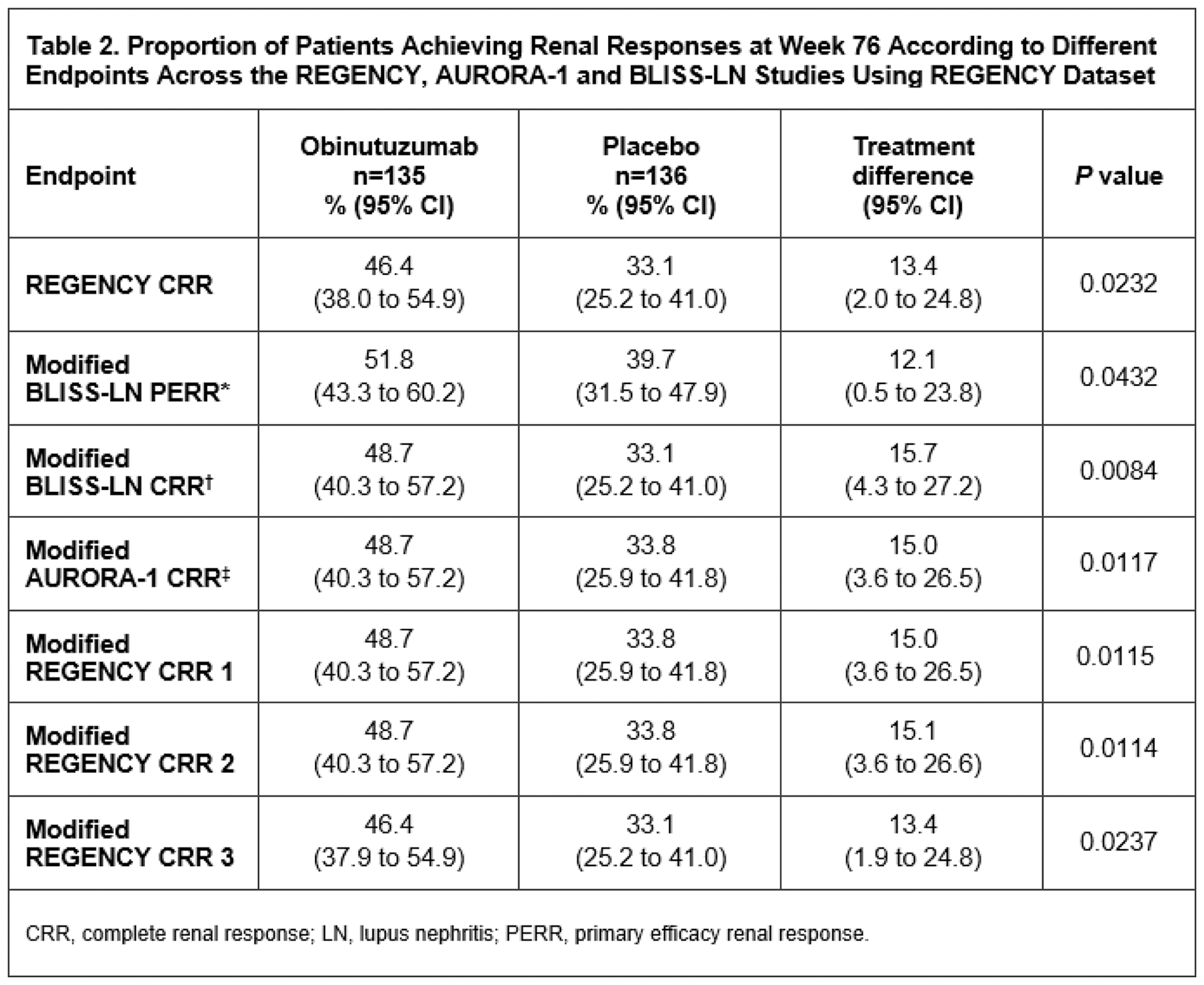
Results: In the obinutuzumab (n=135) plus standard therapy and placebo plus standard therapy groups (n=136), respectively, 46.4% and 33.1% of patients achieved CRR at Week 76 (adjusted difference 13.4%, 95% CI, 2.0 to 24.8%; P =0.0232). The proportions of patients at Week 76 achieving i) a modified BLISS-LN primary efficacy renal response were 51.8% and 39.7% (adjusted difference: 12.1%, 95% CI, 0.5 to 23.8%; P =0.0432); ii) a modified BLISS-LN CRR were 48.7% and 33.1% (adjusted difference: 15.7%, 95% CI, 4.3 to 27.2%; P =0.0084); iii) a modified AURORA-1 CRR were 48.7% and 33.8% (adjusted difference: 15.0%, 95% CI, 3.6 to 26.5%; P =0.0117) (Table 2). Additional modified REGENCY CRR definitions were analysed and summarised in Table 2.
Conclusion: Obinutuzumab plus standard therapy was significantly superior to placebo plus standard therapy across different renal response definitions at Week 76. Consistent benefit was observed, irrespective of the outcome measure composition as well as the stringency of the individual outcome thresholds. Whilst cross-trial comparisons are challenging, a robust treatment benefit of obinutuzumab plus standard therapy appears to be maintained when applying the modified primary and secondary endpoint definitions from the BLISS-LN and AURORA-1 studies. Furthermore, the effect size of the benefit of obinutuzumab over placebo at Week 76 was consistent (between 13% and 16%) using all of the different definitions of response.
REFERENCES: NIL.
Acknowledgements: Funded by F. Hoffmann-La Roche Ltd. Editorial assistance was provided by Nucleus Global, an Inizio company, and funded by F. Hoffmann-La Roche Ltd.
Disclosure of Interests: Brad H. Rovin received consulting fees F. Hoffmann-La Roche Ltd/Genentech, Inc., William F. Pendergraft III shareholder of F. Hoffmann-La Roche Ltd, employee of Genentech, Inc., Liz Lightstone received consulting fees Alexion, AstraZeneca, F. Hoffmann-La Roche Ltd, GlaxoSmithKline, Kezar, Novartis, Otsuka and Pfizer, Zahir Amoura received consulting fees Amgen, AstraZeneca, Genentech, Inc., GlaxoSmithKline, Kezar and Novartis, Richard A. Furie received consulting fees from GlaxoSmithKline and Genentech, Inc., received research support from GlaxoSmithKline and Genentech, Inc., Imran Hassan employee of F. Hoffmann-La Roche Ltd, Bongin Yoo shareholder of F. Hoffmann-La Roche Ltd, employee of Genentech, Inc., Elsa Martins employee of F. Hoffmann-La Roche Ltd, Ann-Christin Hans employee of F. Hoffmann-La Roche Ltd, Theodore A. Omachi shareholder of F. Hoffmann-La Roche Ltd, employee of Genentech, Inc., Thomas Schindler shareholder of F. Hoffmann-La Roche Ltd, employee of F. Hoffmann-La Roche Ltd, Jay Garg shareholder of F. Hoffmann-La Roche Ltd, employee of Genentech, Inc., Jörg Henes received consulting fees from AbbVie, AstraZeneca, BMS, GlaxoSmithKline, Novartis, Pfizer and UCB, Ana Malvar received consulting fees from F. Hoffmann-La Roche Ltd and Genentech, Inc.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (