

Background: Recognition of self-peptides by autoreactive CD4+ T-cells drives systemic autoimmunity. While Sjögren’s syndrome (SjS) and systemic sclerosis (SSc) are both responsive to anti-nuclear antigens (ANAg), local and systemic molecular mechanisms driving distinct tissue pathology remain enigmatic.
Objectives: We aimed to analyze autoreactive CD4+ T-cells across affected tissues and locoregional lymph nodes to identify mechanisms differentiating SjS and SSc, advancing insights into systemic autoimmunity and tissue-specific pathology for personalized therapies.
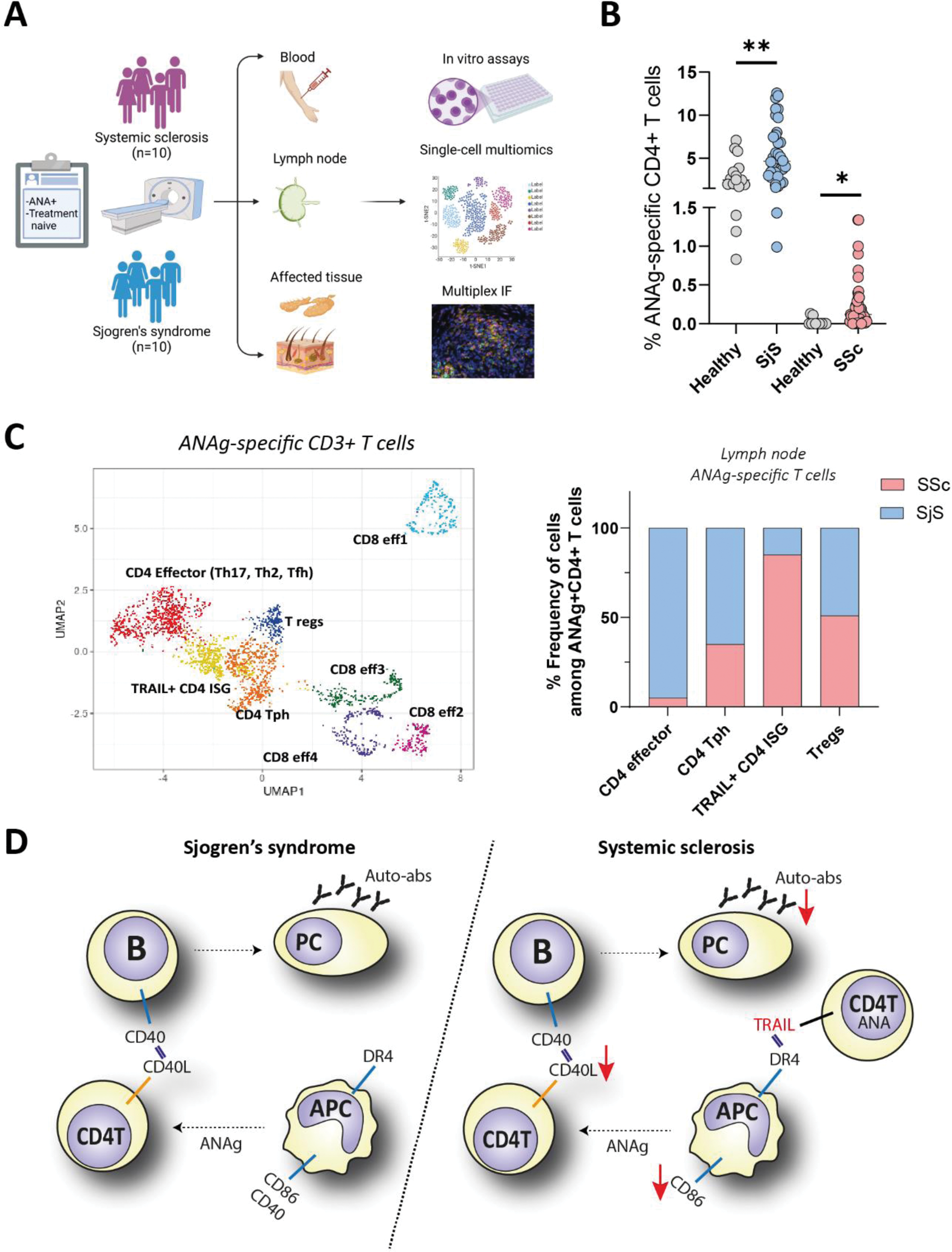
Methods: 3’-fluoro-3’-deoxy-thymidine [18F] FLT PET scans followed by ultrasound-guided biopsies of PET-avid and PET-negative nodes identified reactive lymph nodes (LNs) in SjS (n=10) and SSc (n=10) patients, along with matched affected tissues (salivary gland for SjS, skin for SSc) (Figure 1A). Ex vivo T-cell stimulation assays and multi-modal single-cell analyses (transcriptome, proteome, TCR sequencing) identified ANAg (SSA/SSB and scl70)-reactive CD4+ T-cells in patients’ blood (n=45 SSc, n=37 SjS), LNs and affected tissues (n=10). Flow cytometry and multiplex immunofluorescence validated and mapped the spatial localization of autoreactive cells.
Results: In both SjS and SSc, memory ANAg-reactive T-cell clones were expanded in blood and correlated with disease severity (Figure 1B). Some clones were shared across blood, lymph nodes (LNs), and affected tissues. In SjS, PET-avid LNs and blood showed increased SSA/B-reactive Th2, Th17, follicular, and peripheral T-helper populations compared to PET-negative LNs. In SSc, PET-avid LNs and blood contained primarily scl70-reactive peripheral CD4+ T-cells and few other effector CD4+ T-cells. Notably, a novel CD4+TRAIL+ T-cell population with an interferon stimulated gene signature (ISG) was expanded in PET-avid LNs of SSc patients (Figure 1C). Functionally, this unique T cell population suppressed antigen-presenting cell activation, reducing effector T-helper cell-driven plasma cell formation and autoantibody production (Figure 1D). Strikingly, elevated serum TRAIL levels were linked to milder disease in SSc, highlighting their potentially protective role.
Conclusion: Our data reveal disease-specific autoreactive T-cell immunity. Naive and innate-like CD4+ TRAIL+ ISG ANAg-reactive T-cells differentially regulate ANAg responses in SjS and SSc. The TRAIL-DR4 axis restricts autoantibody production, offering a potential target for developing tolerogenic therapies for systemic autoimmune diseases.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (