

Background: Pulmonary arterial hypertension (PAH) is one of the most severe complications and the leading cause of death in patients with systemic lupus erythematosus (SLE). Therefore, an efficient prognosis stratification instrument and timely intervention in patients with SLE-PAH are in urgent need. Previously, a clinical prognostic model with moderate discrimination (the C-index=0.77) has been established based on eight clinical variables [1]. However, a vital knowledge gap remains regarding the accurate prediction of SLE-PAH prognosis. Such evidence proved that genetic susceptibility may play pivotal roles in the pathogenic mechanisms leading to both SLE and PAH. Therefore, we aimed to evaluate whether genetic variables could refine risk stratification for PAH prognosis beyond the clinical risk prediction model.
Objectives: In a large SLE-PAH prospective cohort, we aimed to investigate the additional role of SLE susceptibility in PAH prognosis prediction.
Methods: Based on the Chinese SLE Treatment and Research Group (CSTAR)-PAH cohort, 273 patients with SLE-associated PAH were recruited. Clinical characteristics and hemodynamic features were recorded, and blood samples were collected for genotyping. SNPs for the genetic risk score (GRS) were identified from the largest meta-analysis of East Asian SLE patients to date [2]. Weighted GRS were assigned to each individual based on 112 non-HLA genetic loci.
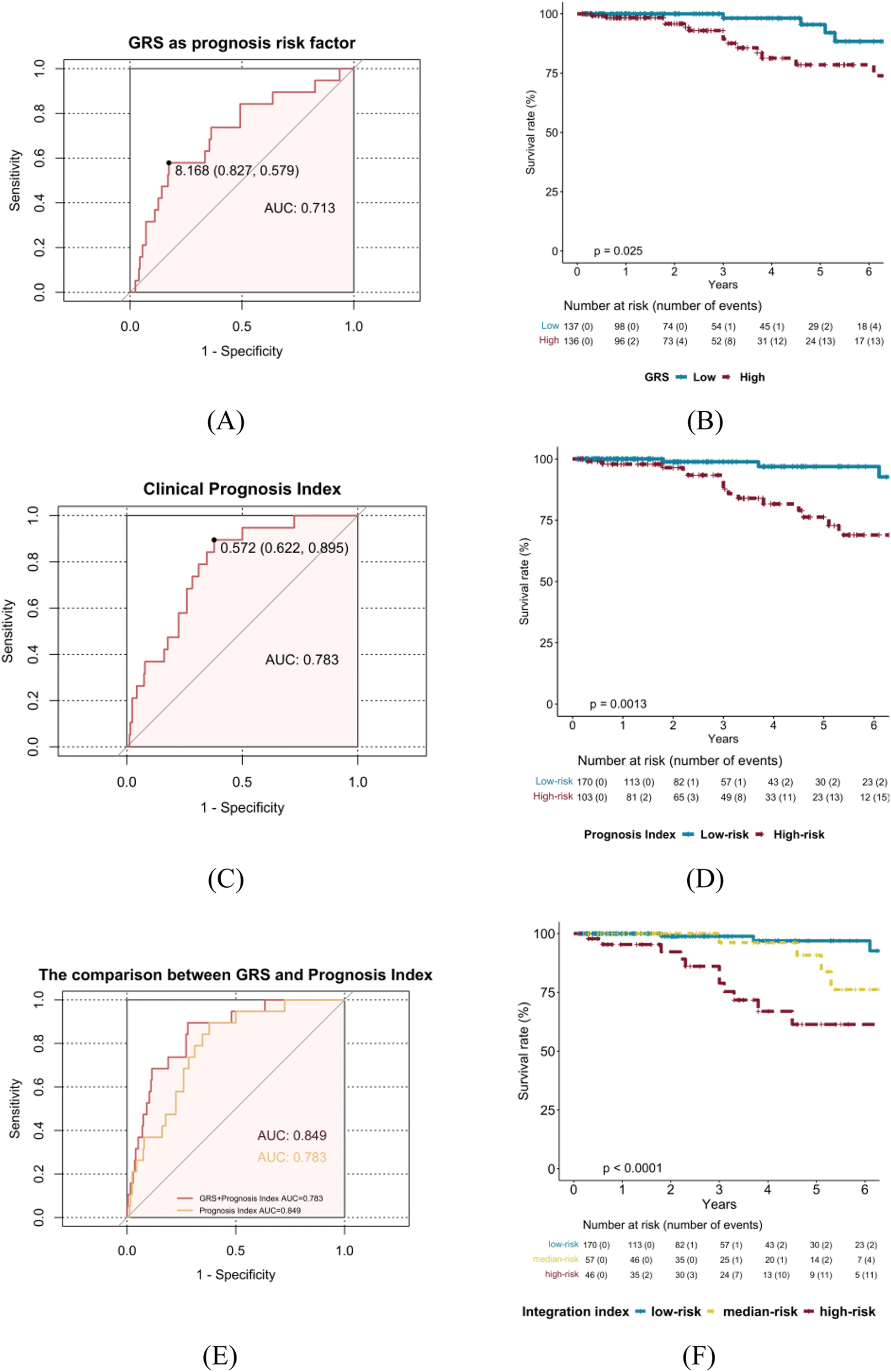
Results: 273 SLE-PAH patients with genotyping data were recruited (Table 1). Among them, 19 (6.9 %) died within a mean follow-up of 3.4 ± 1.8 years. Firstly, a weighted GRS was constructed to assess how the genetic risk affected PAH prognosis. The median (IQR) of GRS was 7.84 (7.47, 8.10) and 136 patients with GRS ≥ 7.84 were considered as the high-GRS group. The Cox regression analysis demonstrated a positive correlation between GRS and mortality (HR=6.82, 95% CI, 2.12-21.91, p=0.001), after adjusting onset age, sex, and disease duration. As shown in Figure 1A and 1B, the ability of GRS to discriminate between SLE-PAH patients with and without poor prognosis was moderate (area under the receiver operating characteristic curve (AUC) = 0.713). Then, we validate the predictive value of a published clinical prognostic index. This clinical prognosis model for SLE-PAH demonstrated good discriminability in our cohort (C-index=0.788, Figure 1C. 103 patients were classified into clinical high-risk group with a survival rate of 85.11%, while 170 were in clinical low-risk group with a survival rate of 97.65% (Figure 1D). Furthermore, we assessed the combined effect of the GRS and clinical prognostic index on PAH mortality. As shown in Figure 1E, integrating of GRS and clinical prognostic index was more predictive than clinical prognostic index alone (AUC=0.849 vs. 0.73). Besides, the combination of genetic and clinical variables was found to be an effective tool for risk stratification. The observed survival rates of SLE-PAH in the clinical low-risk, clinical high-risk with low GRS, and clinical high-risk with high GRS were 97.65%, 92.98%, and 76.09% (Figure 1F). These results suggest that the integration of GRS and clinical prognostic index is indispensable in PAH mortality prediction and stratification.
Conclusion: The GRS could stratify individuals into different trajectories of SLE-PAH prognosis, and further refine mortality risk stratification within each clinical risk stratum, demonstrating a great potential to optimize treatment decisions and improve the prognosis of SLE.
REFERENCES: [1] Qu J, Li M, Zhang X, et al. A prognostic model for systemic lupus erythematosus-associated pulmonary arterial hypertension: CSTAR-PAH cohort study. Respir Res 2023;24(1):220.
[2] Yin X, Kim K, Suetsugu H, et al. Meta-analysis of 208370 East Asians identifies 113 susceptibility loci for systemic lupus erythematosus. Ann Rheum Dis 2021;80(5):632-40. Table 1. Baseline clinical characteristics of the 273 SLE-PAH patients.
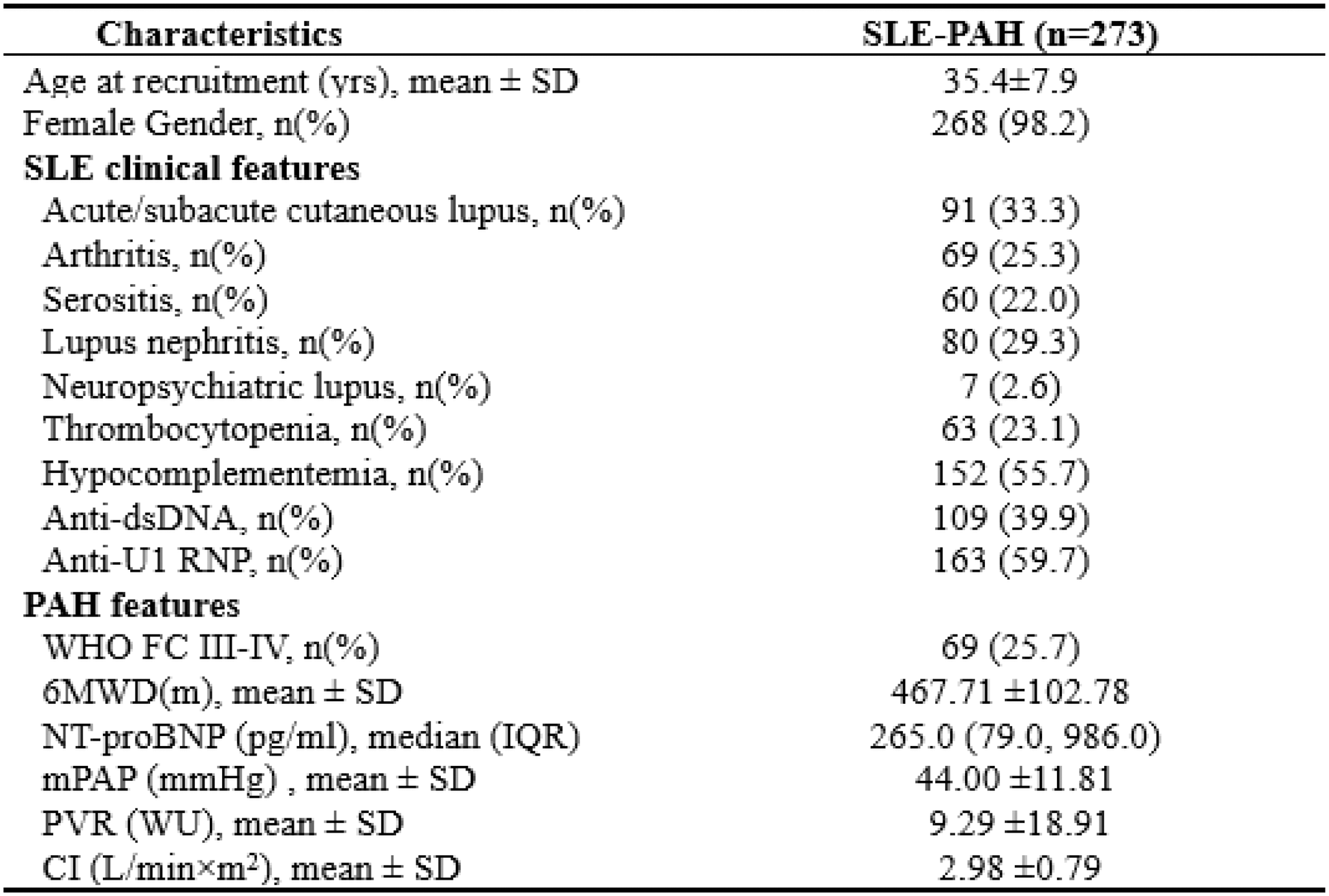
SLE-PAH prognosis stratification based on GRS, prognostic index, and the integration of GRS and clinical risk stratification in 273 SLE-PAH patients. (A) Prediction performance of the weighted GRS; (B) Kaplan-Meier analysis of different risk grades of PAH mortality based on GRS median; (C) Prediction performance of clinical prognostic index; (D) Kaplan-Meier analysis of different risk grades of PAH mortality based on clinical risk categories; (E) Comparison between prediction performance of the GRS and the integration of GRS and clinical prognostic index; (F) Kaplan-Meier analysis of different risk grades of SLE-PAH prognosis based on the integration of GRS and clinical risk stratification.
Acknowledgements: We thank Chinese SLE Treatment and Research Group (CSTAR)-PAH co-authors for assistance with cases collections.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (