

Background: Childhood-onset systemic lupus erythematosus (cSLE) is an autoimmune disease characterised by chronic inflammation and multiple organ damage. Despite their young age, cSLE patients have more significant organ involvement and standardised mortality outcomes compared to adult-onset SLE patients, potentially associated with more pro-inflammatory type I interferons (IFN-I). Understanding the heterogeneity and mechanisms of IFN-I signalling in cSLE will aid personalised therapy and improve patient outcomes.
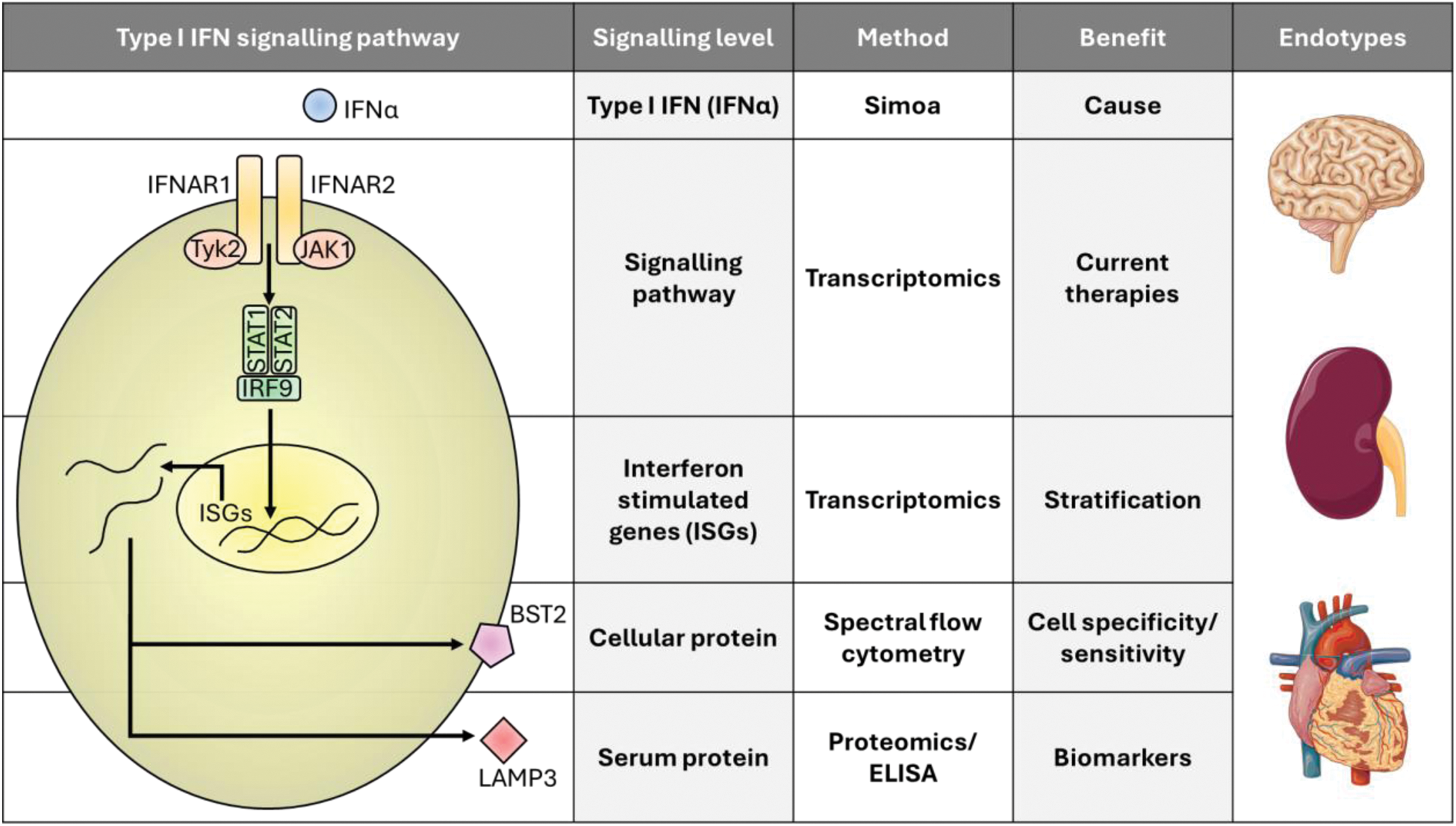
Objectives: For the first time, we used multi-omics to explore IFN-I signatures in cSLE patients in a novel and high resolution approach looking at 4 levels of signalling. This study is extremely important as current anti-IFN therapies target different levels of the signalling cascade, and we are lacking biomarkers to identify patients that would benefit most from these treatments (Figure 1).
Methods: RNA sequencing was used to assess differentially expressed genes (DEGs, Bonferroni correction) in peripheral blood mononuclear cells (PBMCs) from a cohort of cSLE patients (n=74, mean age/disease duration=19/6.5 years) with mixed disease activity scores, as well as age-matched healthy controls (HCs, n=20, mean age=18). Serum pan-IFNα levels were measured by multi-subtype single molecule array (SIMOA). Firefly luciferase reporter cells were used to validate IFNα signalling in serum cultures. BST2 (established IFN-regulated protein) expression was quantified over a 30-parameter immune landscape by spectral flow cytometry and LAMP3 serum protein expression was quantified by Olink proteomics (total 288 proteins) and validated by ELISA. Clinical data was analysed by alluvial plots to determine disease endotypes.
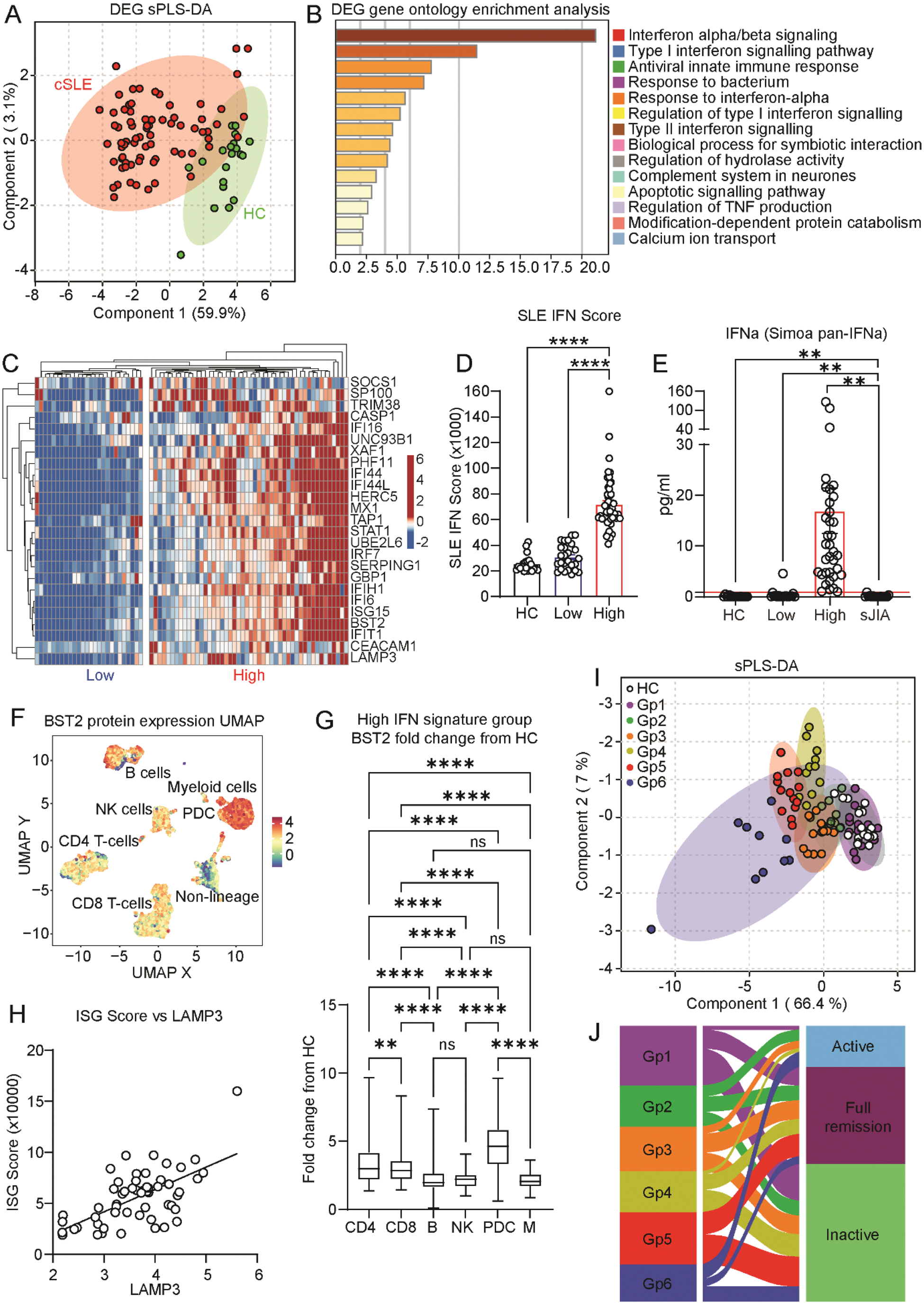
Results: At the gene expression level, 51 DEGs were upregulated in cSLE patients compared to HCs, which together enabled confident separation of the groups by sparse Partial Least Squares Discriminant Analysis (sPLS-DA, Figure 2A). IFN-I signalling was the most significantly enriched pathway in cSLE (p<0.0001, Figure 2B), which was associated with a tight network of other SLE-associated pathways such as apoptosis signalling and complement. Despite this, cSLE patients clustered heterogeneously into high (H-IFN, n=48, 65%) and low (L-IFN, n=26, 35%) IFN-I signature groups (Figure 2C), irrespective of disease activity, which was validated by established SLE IFN-I stimulated gene (ISG) scores (ROC: p<0.0001, AUC=1.00, Figure 2D). Interestingly, the groups persisted in a follow-up timepoint in a subset of the cohort (n=17, ROC: p<0.0034, AUC=0.93), supporting short-term stability. Upstream, H-IFN cSLE patients had significantly higher levels of serum IFNα compared to L-IFN cSLE patients (p= 0.0014), HCs (p= 0.0022), and systemic juvenile idiopathic arthritis (sJIA, n=16) disease controls (p= 0.0018) (Figure 2E) and increased IFNα responsive luminescence following reporter cell culture with H-IFN vs L-IFN patient serum, validating the role of IFNα in driving the groups. Downstream at the cellular protein level, BST2 expression revealed an increased sensitivity to IFN-I signalling in plasmacytoid dendritic cells (PDCs), and CD4+ and CD8+ T cells compared to myeloid cells, B cells, and NK cells (p<0.0001, Figure 2F-G). Extracellularly, proteomics analysis identified serum LAMP3 protein to have the most significant positive correlation with SLE IFN-I scores (p<0.0001, r=0.63, Figure 2H) and the best predictive capacity for the H-IFN group (p<0.0001, AUC=0.95). Biomarker potential was validated over multiple timepoints and by LAMP3 ELISA in matched samples (ROC: p=0.027, AUC=0.89). Combining all multi-level IFN-I signalling data into a normalised clustering approach revealed intra-patient heterogeneity and 6 unique inter-patient cluster groups, differing by serum IFN-I levels, cellular IFN-I and IFN-II scores, sensitivity to IFN-I across cell subsets, and serum LAMP3 expression (Figure 2I). Strikingly, each IFN-I phenotype group had a unique disease endotype and clinical disease activity status (Figure 2J).
Conclusion: This novel multi-level analysis allows us to better understand IFN-I signalling and associated disease endotypes in cSLE (Figure 1). We found that: 1) Patients have heterogeneous IFN-I signatures independent from disease activity; 2) PDCs and T cells may respond more favourably to anti-IFN therapy in specific patients; and 3) LAMP3 is an accurate serum biomarker that could stratify H-IFN patients for therapy. Together, this study represents an important step for personalised IFN-targeted therapeutic approaches in cSLE to improve patient prognosis.
REFERENCES: NIL.
Acknowledgements: All patients and donors involved in this study.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (