

Background: Interstitial lung disease (ILD) remains a leading cause of mortality in systemic sclerosis (SSc) but it is still hard to correctly identify patients at risk of progression. This would avoid overtreatment of non-progressive patients and to closely monitor and rapidly treat those at risk.
Objectives: To build a predictive algorithm that could be used in clinical practice to identify individuals at risk of SSc-ILD progression.
Methods: SSc patients registered in the EUSTAR database, who fulfilled the 2013 ACR/EULAR SSc classification criteria; with ILD by HRCT or X-ray; and with available %pFVC and %pDLCO at baseline and after 12 ± 3 months were included. Patients with baseline %pFVC ≤70 were excluded. ILD worsening was defined as an absolute decline in %pFVC ≥5 (Outcome 1) or ≥10 (Outcome 2) within 1 year. Logistic regression using generalized estimation equations (GEE) and machine learning methods, including gradient boosting, were employed. Model performance metrics were evaluated using ROC curves with area under the curve (AUC) and positive predictive value (PPV). Predictors included demographic (sex, age), baseline serologic (anti-topoisomerase-I [ATA], C-reactive protein), disease specific (disease duration, disease subset, use of proton pump inhibitor) and clinical parameters (%pDLCO, %pFVC, dyspnea, presence of join synovitis, modified Rodnan score).
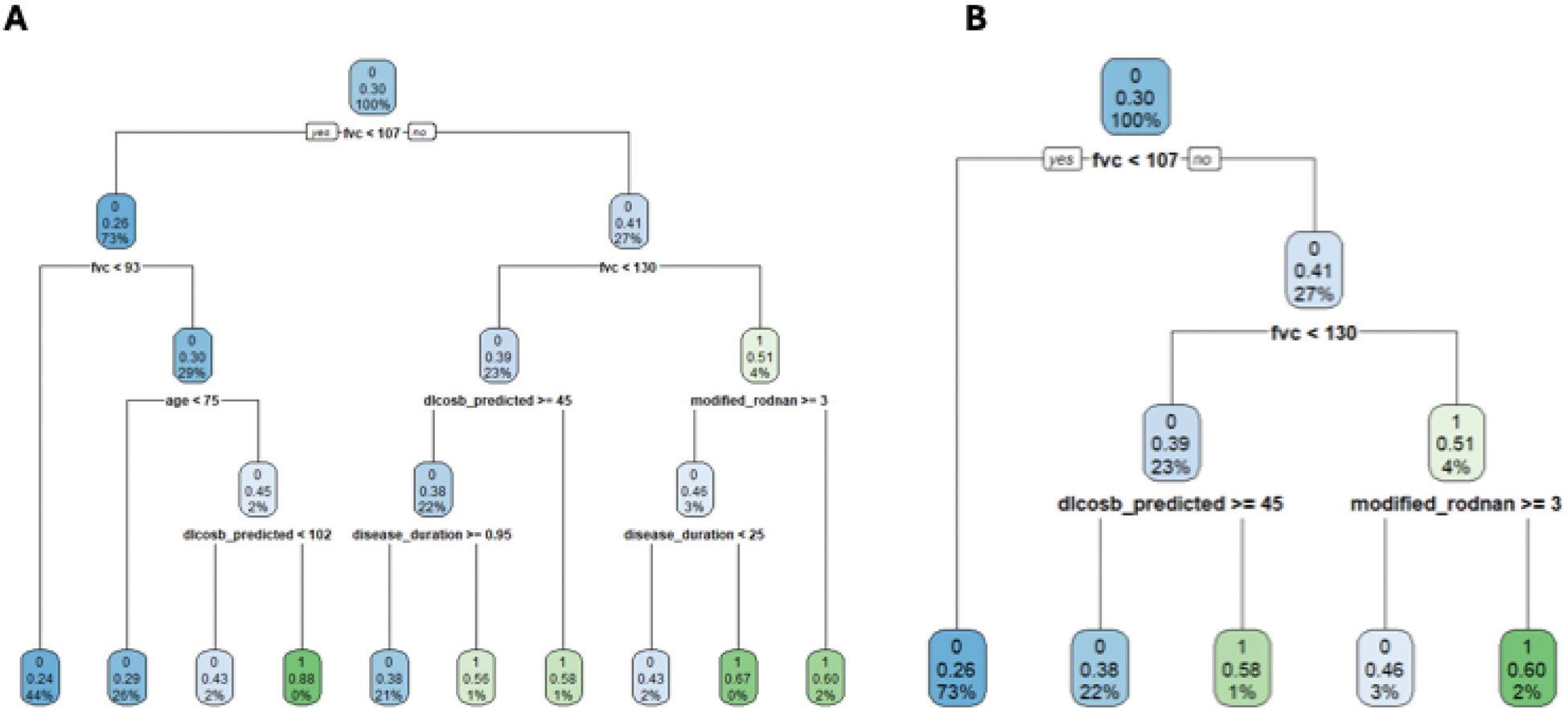
Results: Data from 3281 patients (9,749 one-year periods) were analyzed. Features of worsening and non-worsening patients according to the 2 outcomes are presented in Table 1. All models had similar, relatively low performance. Logistic regression models had a AUC of 0.61 and PPV of 0.34 for Outcome 1, for Outcome 2 AUC was 0.64 and PPV 0.22. Categorized FVC and DLCO with thresholds yielded by 5-level and 4-level simple tree classifications had a similar AUC of 0.60 (Figure 1), a similar performance to advanced machine learning approaches, including gradient boosting and decision trees (AUC ~0.60). Logistic regression (GEE with cluster on the patient, with multiple imputations on the covariates) estimated for Outcome 1: baseline %pFVC (OR per 10-unit increase: 1.02, p<0.001), %pDLCO (OR per 10-unit increase: 0.95, p<0.001), CRP elevation (OR: 1.1, p=0.03), and dyspnea severity (NYHA stage 2: OR 1.2, p=0.02; stage 3: OR 1.5, p<0.001; stage 4: OR 1.8, p=0.04) as significant predictors of worsening. For Outcome 2: baseline %pFVC (OR per 10-unit increase: 1.03, p<0.001), %pDLCO (OR per 10-unit increase: 0.95, p=0.002), and dyspnea severity (stage 3: OR 1.6, p<0.001; stage 4: OR 2.5, p=0.001) as significant predictors.
Conclusion: Baseline FVC, DLCO, dyspnea class, ATA and CRP elevation are key predictors of ILD worsening in SSc. Despite the availability of a large database and advanced predictive models, prediction of ILD progression based on the use of routine collected data is poor. Machine learning provided no significant enhancement compared to logistic regression, supporting that lack of predictivity of the latter is not due to non-linear interactions between covariates, but to lack of information contained in the variable used. Future work should focus on identifying novel risk factors and biomarkers to improve prediction.
Table 1. Features of worsening and non-worsening one-year observation periods according to the 2 outcomes.
Simple tree classification model for SSc-ILD patients, panel A 5-level, panel B 4-level.
REFERENCES: NIL.
Acknowledgements: EUSTAR collaborators.
Disclosure of Interests: Corrado Campochiaro BI, Novartis, J&J, Denis Mongin: None declared, Delphine S Courvoisier: None declared, Giacomo De Luca: None declared, Patricia Carreira Delgado: None declared, Jeska K. de Vries-Bouwstra: None declared, Gábor Kumánovics: None declared, Radim Bečvář: None declared, Oliver Distler 4P-Pharma, Abbvie, Acceleron, Acepodia Biotech, Aera, Alcimed, Altavant, Amgen, AnaMar, Anaveon AG, Argenx, AstraZeneca, Blade, Bayer, Boehringer Ingelheim, Calluna (Arxx), Cantargia AB, Catalyze Capital, Corbus, CSL Behring, Galderma, Galapagos, Glenmark, Gossamer, Horizon, Janssen, Kymera, Lupin, Medscape, MSD Merck, Miltenyi Biotec, Mitsubishi Tanabe, Nkarta Inc., Novartis, Orion, Pilan, Prometheus, Quell, Redxpharma, Roivant, EMD Serono, Topadur and UCB, 4P-Pharma, Abbvie, Acceleron, Acepodia Biotech, Aera, Alcimed, Altavant, Amgen, AnaMar, Anaveon AG, Argenx, AstraZeneca, Blade, Bayer, Boehringer Ingelheim, Calluna (Arxx), Cantargia AB, Catalyze Capital, Corbus, CSL Behring, Galderma, Galapagos, Glenmark, Gossamer, Horizon, Janssen, Kymera, Lupin, Medscape, MSD Merck, Miltenyi Biotec, Mitsubishi Tanabe, Nkarta Inc., Novartis, Orion, Pilan, Prometheus, Quell, Redxpharma, Roivant, EMD Serono, Topadur and UCB, BI, Kymera, Mitsubishi Tanabe, UCB, Alexandra Balbir-Gurman: None declared, Enrico Selvi: None declared, Alberto Cauli: None declared, Simona Rednic: None declared, Francesco Del Galdo Abbvie, AstraZeneca,Arxx, Boehringer-Ingelehim, Chemomab, DeepCure, GSK, Janssen, MistusbishiTanabe, Novartis, Ventus, Abbvie, Argenx, AstraZeneca, Boehringer Ingelehim, Mitsubishi-Tanabe, Ventus, Marco Di Battista: None declared, Irena Litinsky: None declared, Marco Matucci-Cerinic Boehringer Ingelheim, and for consultancies from Chemomab, Astra Zeneca, Boehringer Ingelheim, GSK, Argenx, MSD, Argenx, MSD, Boehringer Ingelheim, Michele Iudici: None declared, Yannick Allanore Abbvie, Alpine ImmunoSciences, Argenx, Astra-Zeneca, Boehringer Ingelheim, Corvus, Merck Serono, Topadur, Abbvie, Alpine ImmunoSciences, Argenx, Astra-Zeneca, Boehringer Ingelheim, Corvus, Merck Serono, Topadur.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (