

Background: Progression of interstitial lung disease (ILD) reduces long-term survival in patients with systemic sclerosis (SSc), therefore frequent monitoring with lung function test for the 1st year, and less frequently once stable is recommended in the 2024 ACR/Chest guidelines. Follow-up HRCT is only recommended as needed. The specific frequency and necessity of follow-up HRCTs is unknown.
Objectives: To assess the impact of annual HRCT monitoring to identify ILD progression and its predictive ability for mortality.
Methods: We included all SSc-ILD patients from two expert centers, having ILD on HRCT and available consecutive annual assessments including HRCT images, forced vital capacity (FVC), diffusing capacity for carbon monoxide (DLCO), respiratory symptoms, and vital status at study end. ILD progression was defined by the 2022 ATS/ERS/JRS/ALAT guideline progressive pulmonary fibrosis (PPF) criteria, with the subcriteria: (1) worsening of respiratory symptoms, (2) absolute decline in FVC ≥5% or in DLCO ≥10%, and (3) disease progression on HRCT assessed by ILD experts, over 12 ±3 months. ILD progression on HRCT was evaluated by experts, using the definition provided by the guideline. We assessed the prevalence of PPF and of the subcriteria comprising the definition, then applied Cox regression to identify their impact on mortality. Kaplan Meier estimates were used to analyze 3-year survival.
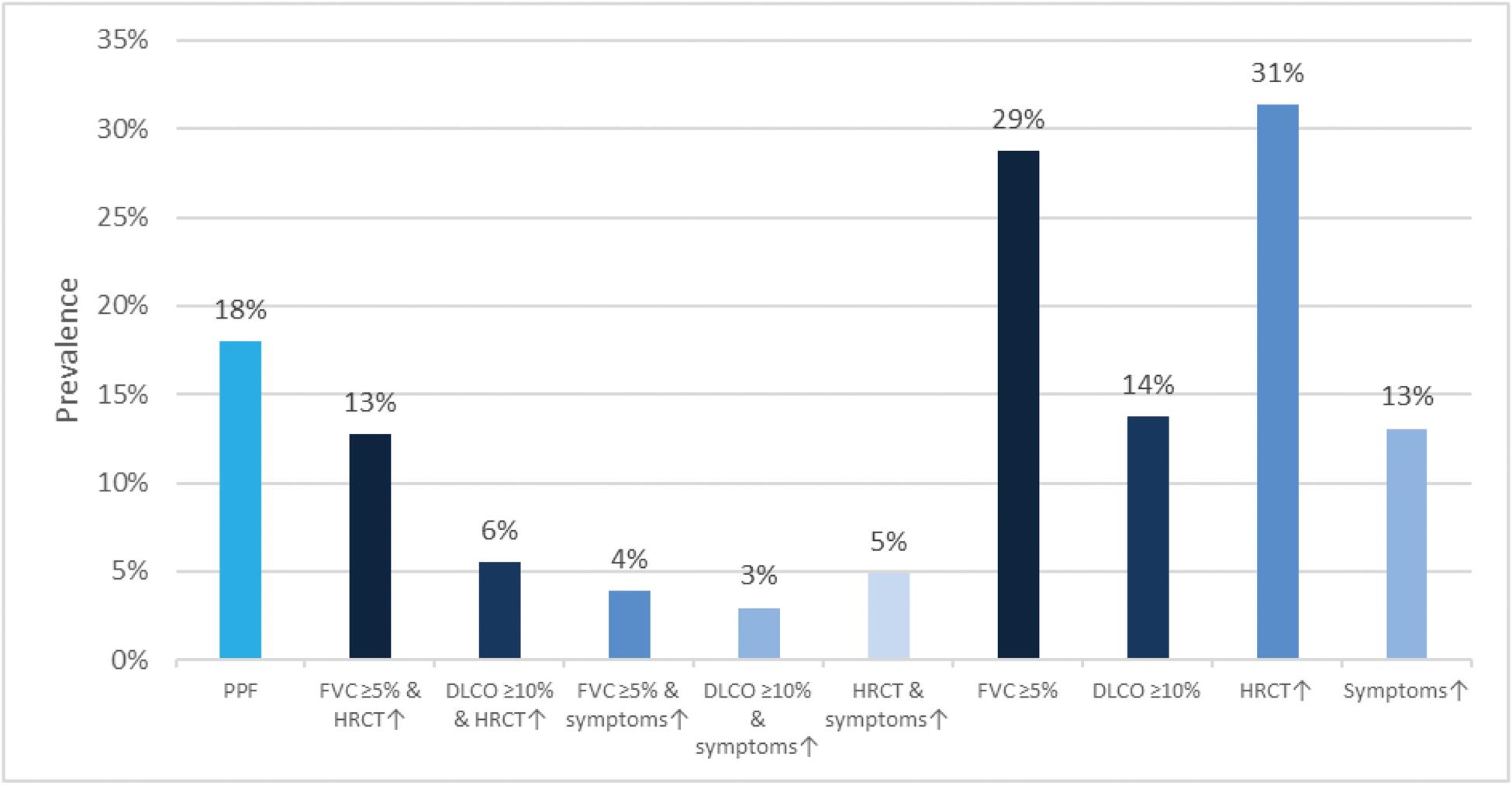
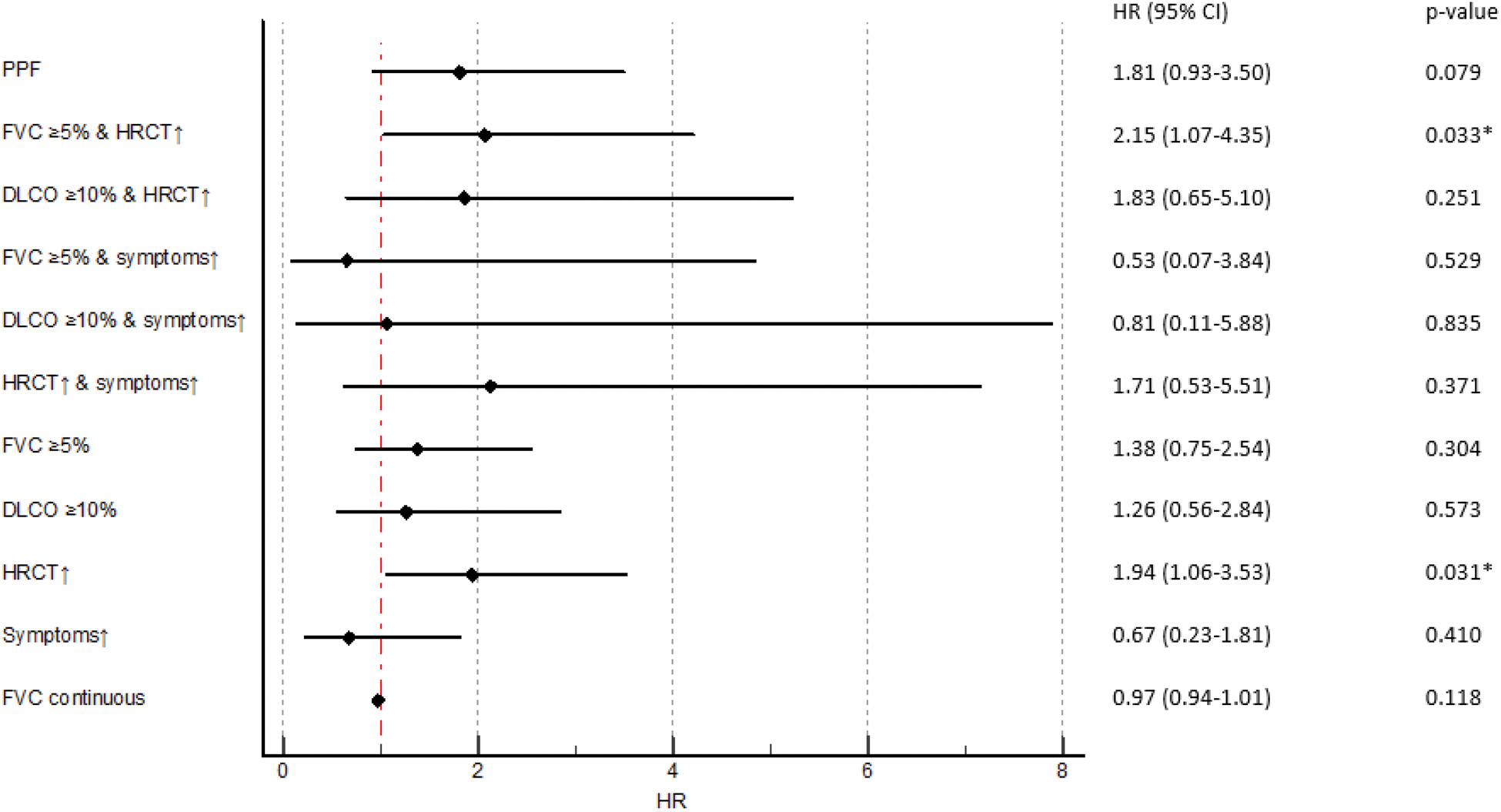
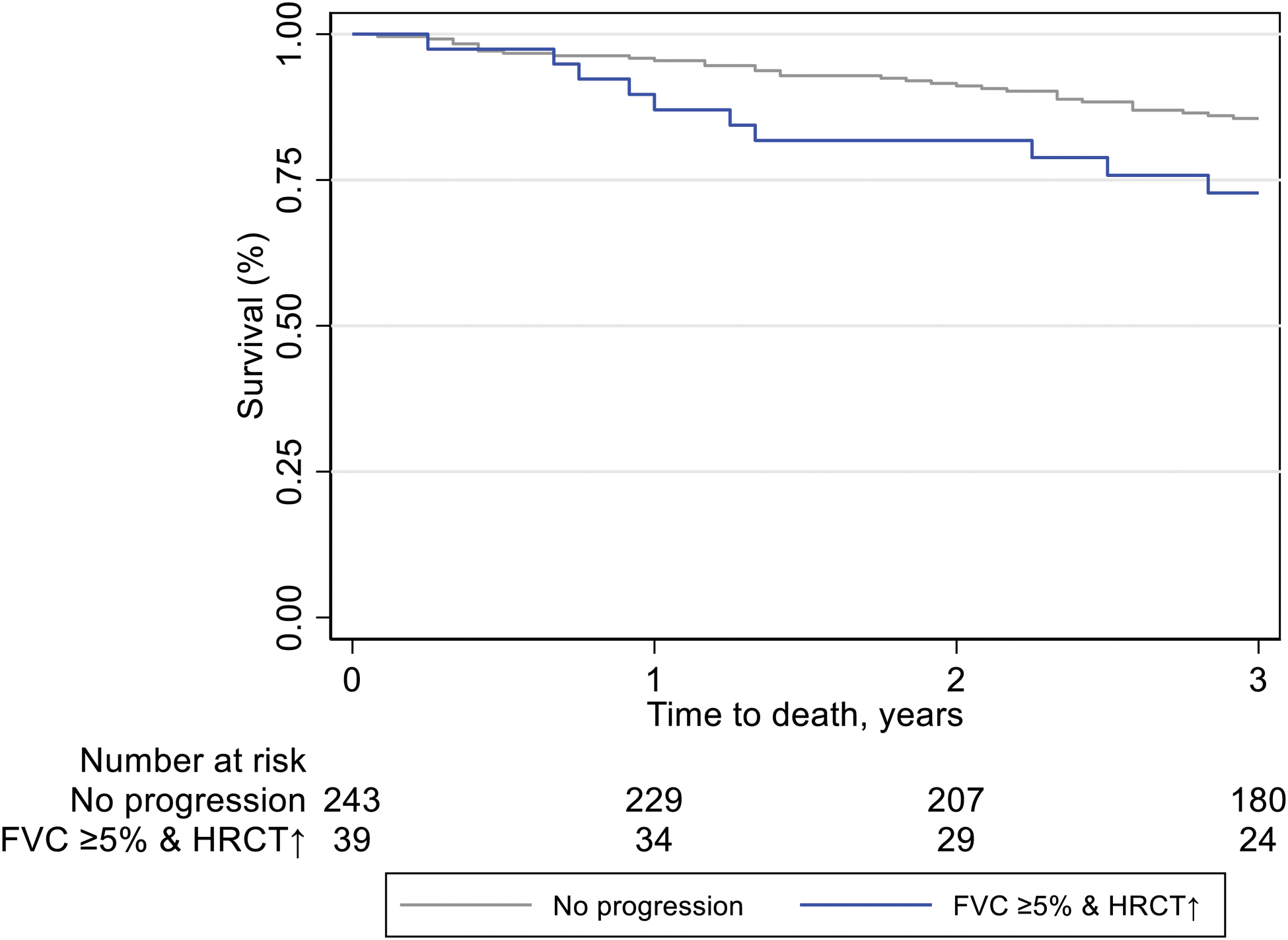
Results: We identified 306 SSc-ILD patients mirroring a typical SSc-ILD population, with 74 (24%) males, 120 (39%) with diffuse SSc, 114 (37%) with anti-topoisomerase I antibodies, mean disease duration of 9.3±11.3 years, mean FVC of 87%±19.8, DLCO of 65%±19.6 and 81 (28%) with >20% of fibrosis on HRCT. PPF was detected in 55 (18%) SSc-ILD patients over 12 ±3 months. Fulfilment of the PPF criteria was mainly driven by HRCT and pulmonary function results: specifically, by the combination of absolute FVC decline ≥5% and worsening on HRCT (39/55, 71%), followed by absolute DLCO decline ≥10% and worsening on HRCT (17/55, 31%) and by worsening on HRCT and respiratory symptoms (15/55, 27%) (Figure 1A). Next we assessed the impact of PPF and its subcategories on mortality and determined which specific criteria had the strongest impact. Over mean 3.5 ±0.87 years, 45 (15%) patients died, with 12 (27%) showing PPF over the first 12 months. While PPF itself was not associated with mortality, the subcriteria of FVC ≥5% decline in combination with worsening on HRCT and worsening on HRCT alone did significantly predict mortality (Figure 1B). PPF showed no significant association with 3-year-mortality, but the combination of absolute FVC decline ≥5% and disease progression on HRCT was significantly reducing the 1- and 3-year survival with p=0.039 (Figure 1C).
Conclusion: Worsening on HRCT is the driving subcriterion of the PPF criteria and the strongest predictor for reduced survival. Annual HRCT is therefore of high importance for timely identification of ILD progression in SSc and for assessing the risk of poor disease outcome. Not using HRCT may lead to missed opportunities for optimized disease management.
REFERENCES: NIL.
Prevalence of PPF and its subcriteria in SSc-ILD
The impact of PPF and its subcriteria on mortality, assessed by cox regression
The impact of FVC decline and worsening of ILD on HRCT (blue) compared to no worsening (grey) on 3-year survival assessed by Kaplan-Meier estimates (p=0.039)
Acknowledgements: NIL.
Disclosure of Interests: Anna-Maria Hoffmann-Vold Boehringer Ingelheim, Janssen, Medscape, Merck Sharp & Dohme, Novartis and Roche, AbbVie, ARXX, Boehringer Ingelheim, Bristol Myers Squibb, Genentech, Janssen, Medscape, Merck Sharp & Dohme, Pliant Therapeutics, Roche and Werfen, Boehringer Ingelheim, Janssen, Liubov Petelytska Swiss National Research Foundation/Scholars at risk, Håvard Fretheim Boehringer Ingelheim, payments made to his institution, Trond Mogens Aaløkken: None declared, Mike O. Becker Vifor, Novartis, GSK, Novartis Foundation for Bio-Medical Research, Foundation for research in Rheumatology (FOREUM). Congress Support from Vifor, GSK, Hilde Jenssen Bjørkekjær Janssen, Cathrine Brunborg: None declared, Dr. Cosimo Bruni Boehringer Ingelheim, Grant/research support from: Novartis Foundation for medical-biological research, EMDO Foundation, Iten-Kohaut Foundation. Educational grants from Wellcome Trust. Congress support from Boehringer-Ingelheim, Hartmann-Muller Foundation, Christian Clarenbach Boehringer Ingelheim Roche, Phuong Phuong Diep Boehringer-Ingelheim, NordicInfu Care AB, Boehringer-Ingelheim, Rucsandra Dobrota: None declared, Michael T Durheim: None declared, Muriel Elhai Boehringer Ingelheim, Grant/research support from Pfizer, Novartis Foundation for Bio-Medical Research, Iten Kohaut foundation, Kurt und Senta Herrmann foundation, Foundation for research in Rheumatology (FOREUM), University Zurich, Walter and Gertrud Siegenthaler Foundation, Theodor und Ida Herzog-Egli – Stiftung and Association des Sclérodermiques de France (ASF). Congress Support from Astrazeneca and Janssen, Thomas Frauenfelder Bayer, Emily Langballe Boehringer Ingelheim, Øyvind Midtvedt Boehringer Ingelheim paid to the institution, Natasha Moe: None declared, Carina Mihai MED Talks Switzerland, Mepha, MedTrix, Novartis, PlayToKnow, Boehringer Ingelheim und Janssen, Congress support from Boehringer Ingelheim, Adela-Cristina Sarbu: None declared, Marco Sprecher AbbVie, Øyvind Molberg: None declared, Oliver Distler 4P-Pharma, Abbvie, Acceleron, Acepodia Biotech, Aera, Alcimed, Altavant, Amgen, AnaMar, Anaveon AG, Argenx, AstraZeneca, Blade, Bayer, Boehringer Ingelheim, Calluna (Arxx), Cantargia AB, Catalyze Capital, Corbus, CSL Behring, Galderma, Galapagos, Glenmark, Gossamer, Horizon, Janssen, Kymera, Lupin, Medscape, MSD Merck, Miltenyi Biotec, Mitsubishi Tanabe, Nkarta Inc., Novartis, Orion, Pilan, Prometheus, Quell, Redxpharma, Roivant, EMD Serono, Topadur and UCB, BI, Kymera, Mitsubishi Tanabe, UCB; Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143). Co-founder of CITUS.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (