

Background: In clinical trials involving patients with psoriatic arthritis (PsA), plaque psoriasis or axial spondyloarthritis treated with bimekizumab (BKZ), a monoclonal IgG1 antibody that selectively inhibits interleukin (IL)-17F in addition to IL-17A, consistent levels of treatment response have been observed regardless of prior tumour necrosis factor inhibitor (TNFi) exposure [1-5]. This is notable as clinical trial and registry data from patients with PsA treated with other biologic disease-modifying antirheumatic drugs (bDMARDs), including IL-17A inhibitors, showed that TNFi-naïve patients were more likely to achieve treatment response targets than TNFi-experienced patients [6,7].
Objectives: To test the hypothesis that immune signalling pathways, specifically IL-17F signalling, may be differentially modulated following treatment with TNFi, providing a potential mechanism for the consistent level of clinical response observed with BKZ treatment across bDMARD-naïve and TNFi-experienced patients with PsA.
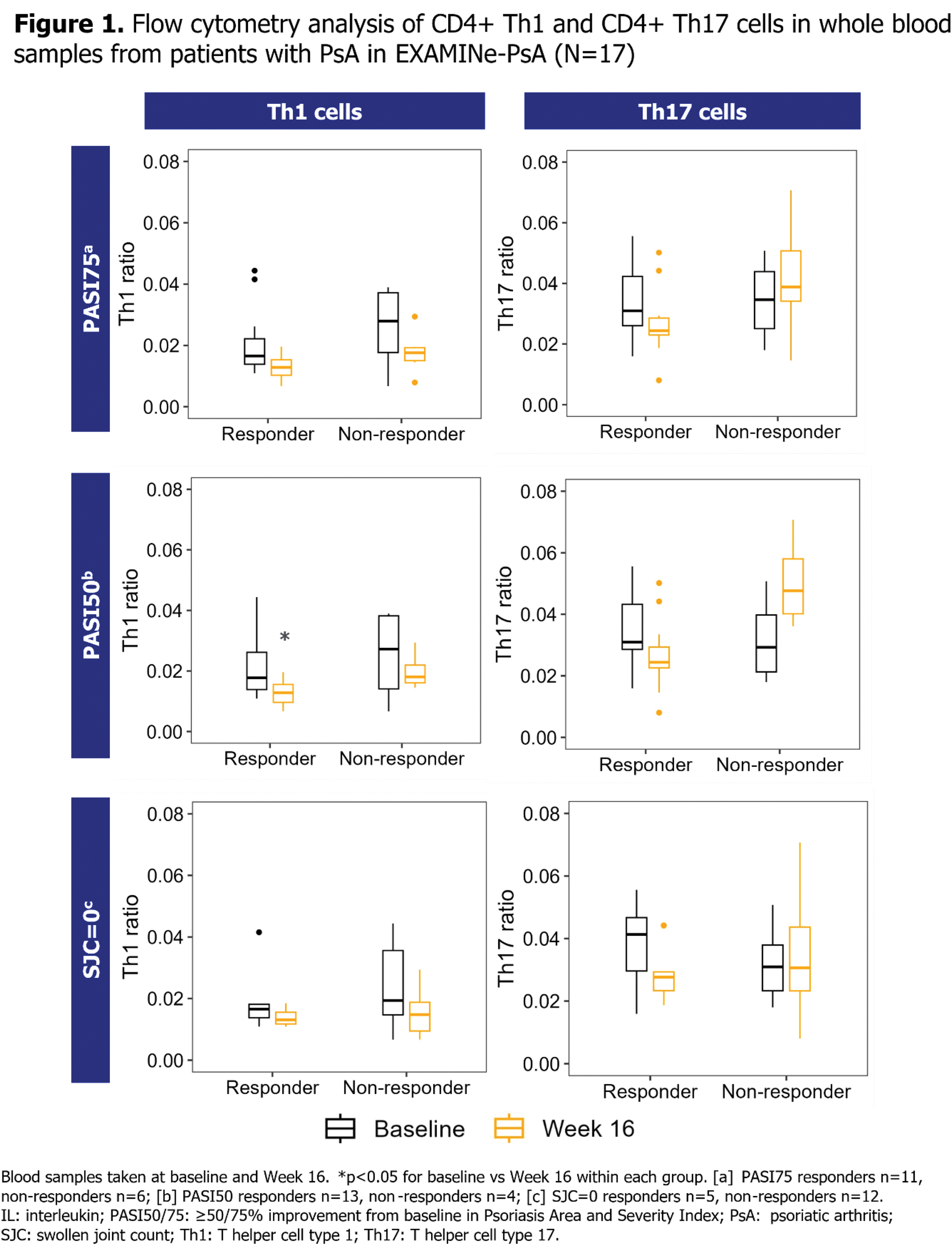
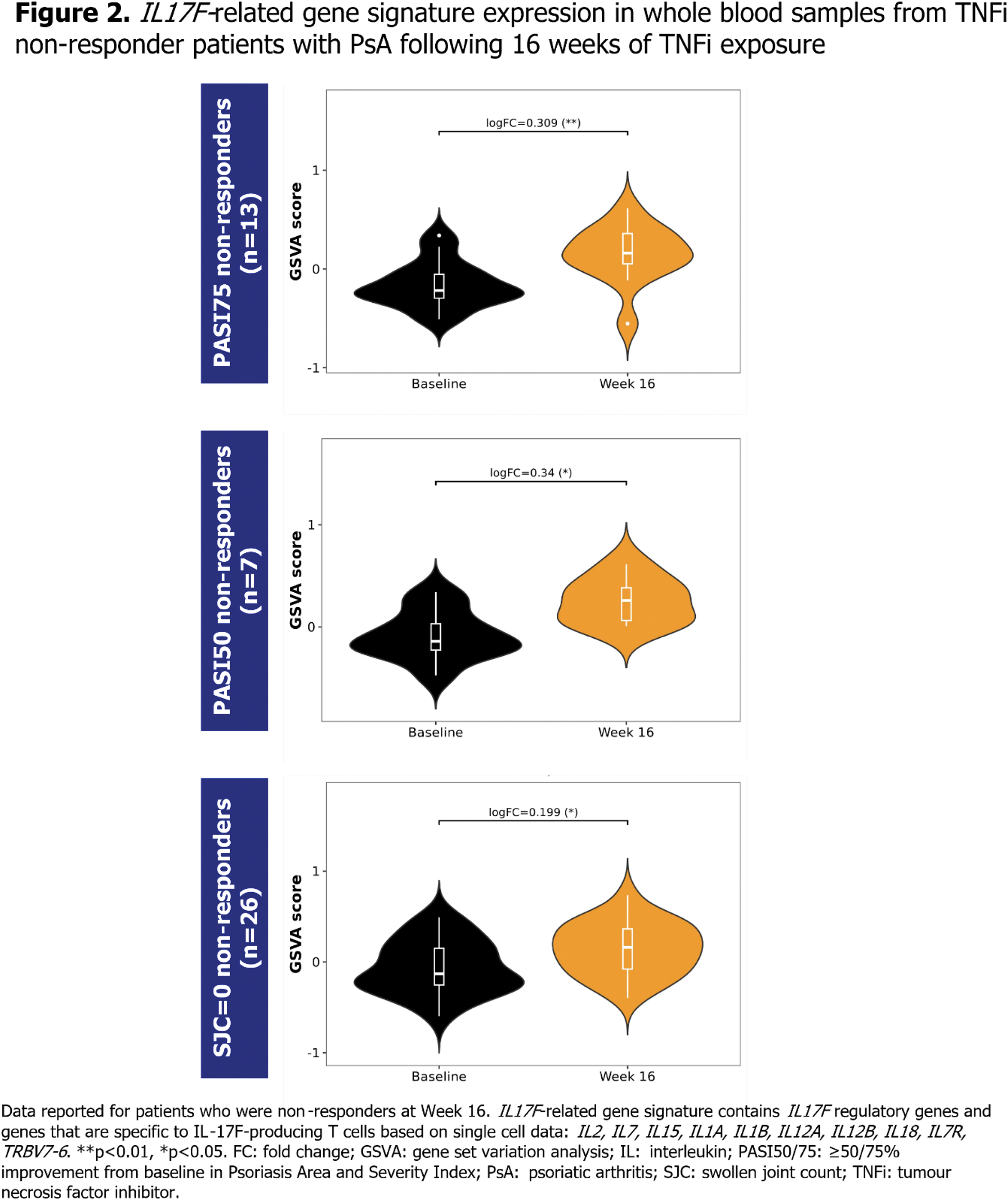
Methods: Immune signalling was assessed in two separate studies of bDMARD-naïve patients with PsA initiating their first TNFi treatment, by examining immune cell composition and IL17F -related gene signatures, respectively. For the purpose of these analyses, patients were defined as TNFi non-responders if they failed to achieve Psoriasis Area and Severity Index (PASI) ≥75% improvement from baseline (PASI75), PASI ≥50% improvement from baseline (PASI50) or resolution of swollen joint count (SJC=0) at Week 16. EXploring Autoimmune disease Mechanisms IN Psoriatic Arthritis (EXAMINe-PsA) assessed a cohort of bDMARD-naïve patients initiating TNFi treatment in the real world [8], to examine immune cell composition changes in TNFi responders vs non-responders. Patients in this cohort initiated various TNFi treatments in the course of routine clinical practice. Flow cytometry was performed on whole blood samples taken at baseline and at Week 16 of TNFi exposure, and the number of CD4+ T helper cell type 1 (Th1) and CD4+ T helper cell type 17 (Th17) cells was evaluated, defined by measurement of cell surface markers (Th1: CD3+CD4+CXCR3+CCR6-; Th17: CD3+CD4+CXCR3-CCR6+) [9]. In a separate analysis, IL17F -related gene expression was assessed in bDMARD-naïve patients who initiated treatment with a TNFi, adalimumab (ADA), and were subsequently TNFi non-responders at Week 16. This was conducted as a post hoc biomarker analysis of samples collected during BE OPTIMAL (NCT03895203), a phase 3 study of bDMARD-naïve patients with PsA [1]. Bulk RNA sequencing (RNA-seq) was performed on whole blood samples taken at baseline and Week 16. Geneset level expression analyses for IL17F -related gene signatures were performed using Gene Set Variation Analysis (GSVA) and limma statistical methods [10,11].
Results: Analyses of immune cell composition in blood samples in EXAMINe-PsA suggested a trend towards increased levels of circulating Th17 cells at Week 16 compared with baseline levels in PASI50 non-responders, albeit this was not a significant increase (Figure 1). There was a less pronounced increase in circulating Th17 cells in PASI75 non-responders. For SJC=0 non-responders, no change in Th17 cell levels was observed. There was no increase in circulating Th17 cells in TNFi responders, and no increase in circulating Th1 cells observed in any group. IL17F gene expression was not detectable in whole blood samples from patients receiving ADA by bulk RNA-seq; therefore, IL17F -related signatures, comprised of regulatory genes and genes specific to IL17F -expressing T cells [12,13], were used to determine possible immune priming indicative of IL17F expression in tissue. GSVA analysis showed that TNFi non-responders had a significant increase in an IL17F -related gene signature at Week 16 compared with baseline (Figure 2).
Conclusion: Following treatment with TNFi, skin and joint non-responders had increased expression of the IL17F -related gene signature. This provides indirect evidence that IL-17F levels may increase following TNFi exposure in non-responder patients. These data suggest that IL-17F is pivotal in driving the pathobiology of PsA, and that its expression may be dynamically regulated. Our results provide a potential explanatory mechanism for the consistent level of clinical response observed with BKZ treatment in TNFi-experienced and bDMARD-naïve patients in phase 3 clinical trials. Whilst numerical differences in immune cell composition were observed in TNFi responders vs non-responders, these were not significant. Therefore, further analyses of IL-17F production and activity at sites of inflammation in TNFi-experienced patients with PsA are required.
REFERENCES: [1] McInnes IB. Lancet 2023;401:25–37.
[2] Merola JF. Lancet 2023;401:38–48.
[3] Mease PJ. Rheumatol Ther 2024;11:1363–82.
[4] Strober B. JAAD 2024;91:Supplement (Abstract 52675).
[5] Magrey M. Ann Rheum Dis 2023;82:876–77 (Abstract POS1107).
[6] Xie Y. Clin Exp Dermatol 2022;47:1627–35.
[7] Merola JF. Semin Arthritis Rheum 2017;47:29–37.
[8] Højgaard P. BMJ Open 2016;6:e010650.
[9] Maecker HT. Nat Rev Immunol 2012;12:191–200.
[10] Hänzelmann S. BMC Bioinformatics 2013;14:7.
[11] Ritchie ME. Nucleic Acids Res 2015;43:e47.
[12] Cutcutache I. ISDS 2023 (Poster 187).
[13] Cole S. J Allergy Clin Immunol 2023;152:783–98.
Acknowledgements: Funded by UCB. Medical writing support provided by Costello Medical and funded by UCB. Iain B. McInnes and Ioana Cutcutache jointly contributed to this study.
Disclosure of Interests: Iain B. McInnes Consulting fees and honoraria from AbbVie, AstraZeneca, BMS, Boehringer Ingelheim, Cabaletta, Causeway Therapeutics, Celgene, Eli Lilly and Company, Evelo, Janssen, MoonLake Immunotherapeutics, Novartis and UCB, Research support from BMS, Boehringer Ingelheim, Celgene, Janssen, Novartis and UCB, Ioana Cutcutache Shareholder of UCB, Employee of UCB, Leon Eyrich Jessen Acted as a paid instructor via role as co-founder and co-owner of a small consultancy (nordicdatalab), Acted as a speaker via role as co-founder and co-owner of a small consultancy (nordicdatalab), Shareholder of Bavarian Nordic and Novo Nordisk, Previously employed at Steno Diabetes Center (Novo Nordisk Foundation), Acted as consultant via role as co-founder and co-owner of a small consultancy (nordicdatalab), Magnus Blom Petersen Student internship at LEO Pharma, Research support from LEO Pharma, Melanie Randahl Nielsen: None declared, Marie Skougaard Speaker fees from Janssen-Cilag, Research funding from Eli Lilly, Pfizer and UCB, Victoria Svinti MacLeod Employee of UCB, Andrew Skelton Shareholder of UCB, Employee of UCB, Adam Prickett Shareholder of UCB, Employee of UCB, Stevan Shaw Shareholder of UCB, Employee of UCB.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (