

Background: Paget’s disease of bone (PDB) is a late-onset skeletal disorder characterized by excessive bone resorption and disorganized remodeling. Mutations in the ZNF687 and PFN1 genes have been identified in severe forms of early-onset PDB, which, in rare cases, can progress to osteosarcoma or other malignancies. However, the specific effects of these genetic alterations on osteoclast biology and skeletal homeostasis are poorly defined.
Objectives: This study aimed to investigate the roles of ZNF687 and PFN1 mutations in regulating bone marrow-derived osteoclast progenitors, macrophages, osteoclast activity, and bone mass, shedding light on their contributions to PDB pathogenesis and potential progression to malignancy.
Methods: Mouse Models : Zfp687 P937R knock-in ( Zfp687 P937R/P937R ), Zfp687 knock-out ( Zfp687 -/- ), and Pfn1 c.318_321del knock-in ( Pfn1 c.318_321del/WT ) mice were studied; Cell and Bone Analysis : Bone marrow-derived osteoclast progenitors (CD117+B220-CD115+CD11b-), and macrophages (CD45+CD11b+F4/80+) were quantified using flow cytometry. Osteoclast activity was histologically assessed via TRAP-staining. Trabecular bone mass of the femurs and L4 vertebrae was evaluated using micro-computed tomography (µCT).
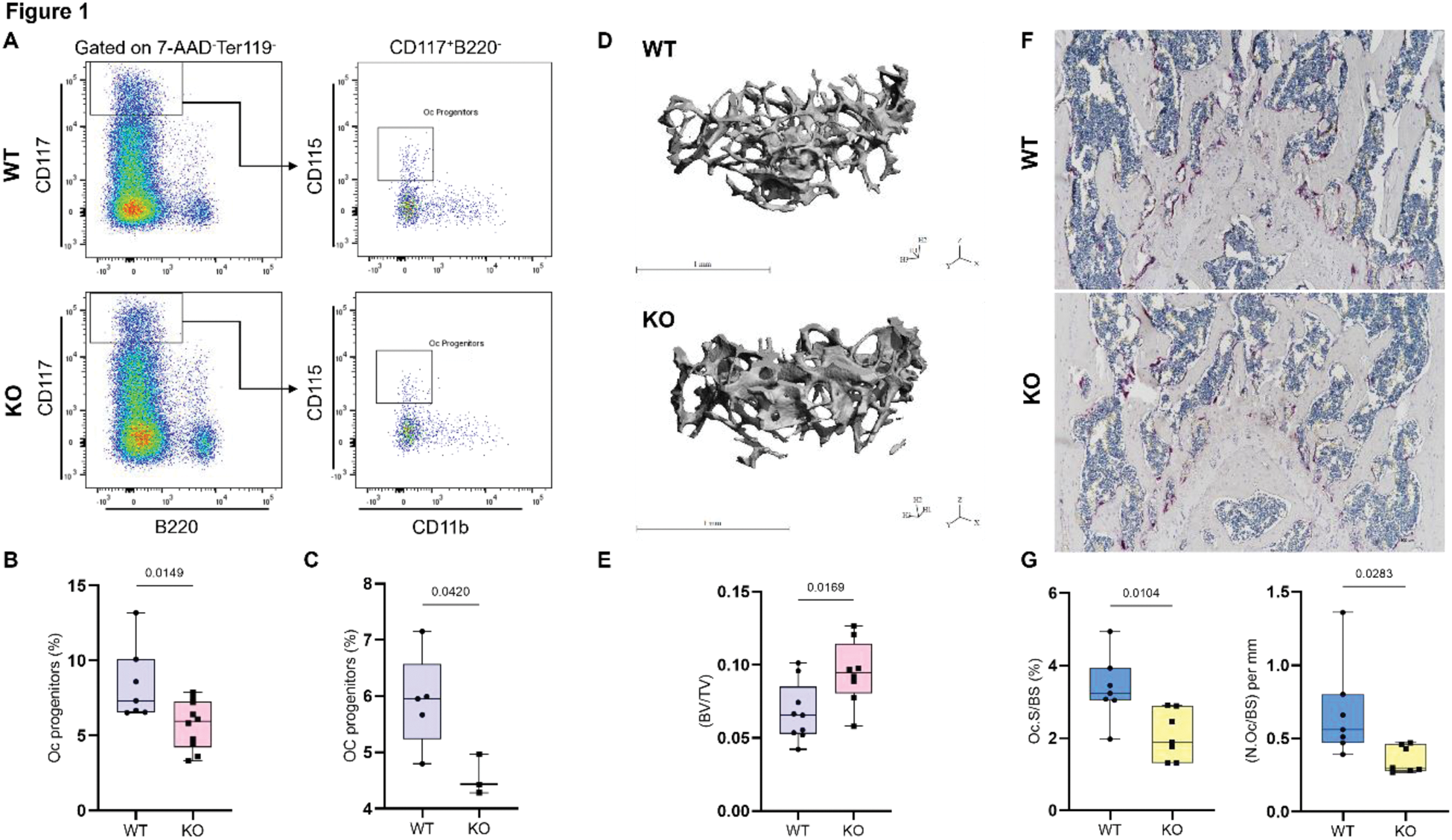
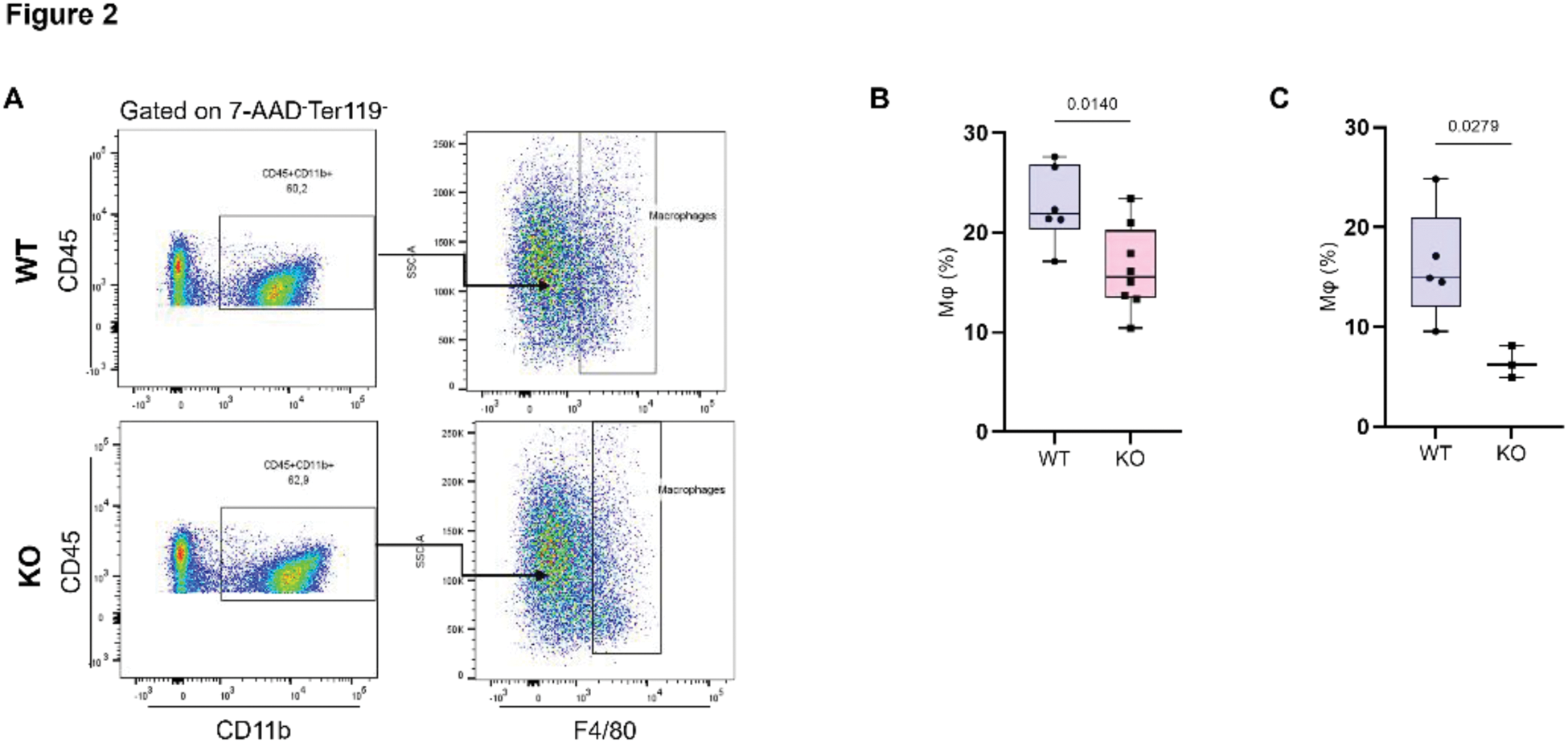
Results: Zfp687 -/- mice exhibited significantly reduced osteoclast progenitors from 3 months of age, a reduction that persisted at 8 months ( p =0.0149; p =0.0420, respectively) (Figure 1 a;b;c). This corresponded to an increased trabecular bone mass in the femurs of 8-month-old mice ( p =0.0169) (Figure 1d;e). Histological analysis of TRAP-stained femur sections confirmed impaired osteoclast activity, with decreased osteoclast surface and number per bone surface as early as 3 months of age ( p =0.010; p =0.028) (Figure 1f;g). The reduced levels of osteoclast progenitors were also paralleled by decreased macrophage levels at both 3 and 8 months of age ( p =0.0140; p =0.0279), highlighting the importance of ZNF687 in regulating myeloid lineage populations critical for osteoclastogenesis. Conversely, Zfp687 P937R/P937R mice showed elevated macrophage levels compared to wild-type controls ( p =0.0434), supporting previously published data linking this mutation to increased osteoclast activity and reduced bone mass in both the axial and the appendicular skeleton, closely mimicking PDB onset at 8 months of age. Although the PFN1 mutation is known to contribute to PDB, Pfn1 c.318_321del/WT mice displayed only a positive trend, without significant alterations in osteoclast progenitors or macrophage levels, suggesting a finer regulatory role.
Conclusion: These findings reveal that ZNF687 mutations exert distinct effects on osteoclastogenesis and skeletal remodeling. Specifically, the loss of Zfp687 results in the disruption of progenitor development and increases bone mass. In contrast, the P937R mutation leads to excessive bone resorption and pathological remodeling, characteristic of PDB. On the other hand, the PFN1 mutation does not significantly alter osteoclast progenitor development, despite its well-established role in osteoclast biology. Together, these results underscore the critical and divergent roles of ZNF687 and PFN1 in bone biology, highlighting their potential as therapeutic targets in PDB and related disorders.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (