

Background: Gout due to the presence of monosodium urate crystals (MSU) which occurs after chronic hyperuricemia is responsible for recurrent inflammatory flares. Gout inflammation is driven by IL-1β release after interaction of MSU crystals with immune cells. Gout flare is characterized by a self-limited inflammatory reaction. The mechanisms of crystal-induced inflammation initiation remain unclear. We recently observed the increase of several inflammatory biomarkers including TNFSF14 and IL-6 during gout flare in a prospective gout cohort (GOUTROS).
Objectives: Our objective is to analyze and identify metabolites associated with gout flare and resolution in a prospective gout cohort.
Methods: Plasmas were collected from 71 patients (5 females and 66 males) during gout flare (T1) and after the flare (T2) and analyzed by Nuclear Magnetic Resonance (NMR) based metabolomic. A total of 146 parameters (115 Lipoproteins and 41 Metabolites) were quantified using a targeted approach. Glutamine and glutamate were measured in the synovial fluid of patients suffering from osteoarthritis (OA), gout and calcium pyrophosphate deposition disease by colorimetric dosage (Sigma). THP-1 monocytes were primed by PMA (phorbol 12-myristate 13-acetate) and stimulated by synthetic MSU crystal in the presence or not of metabolite (glutamine or histidine) supplementation.
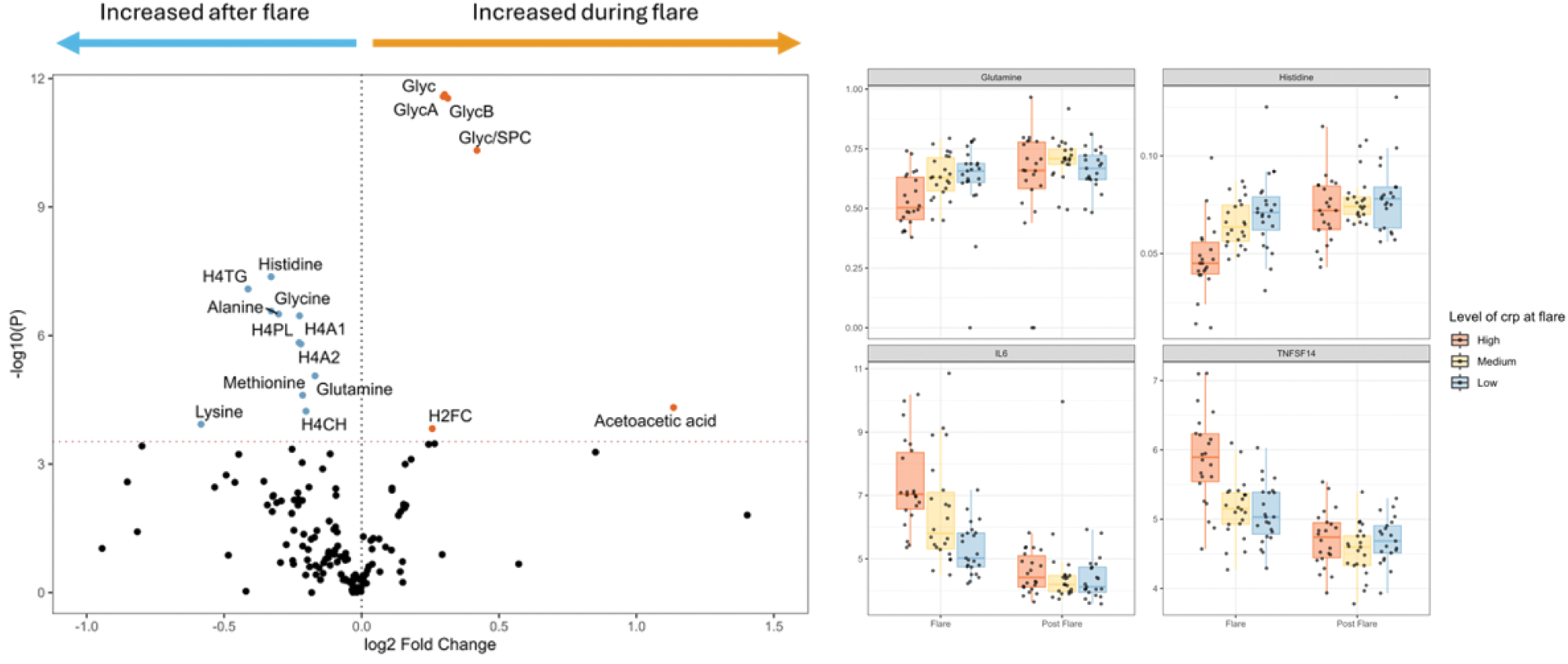
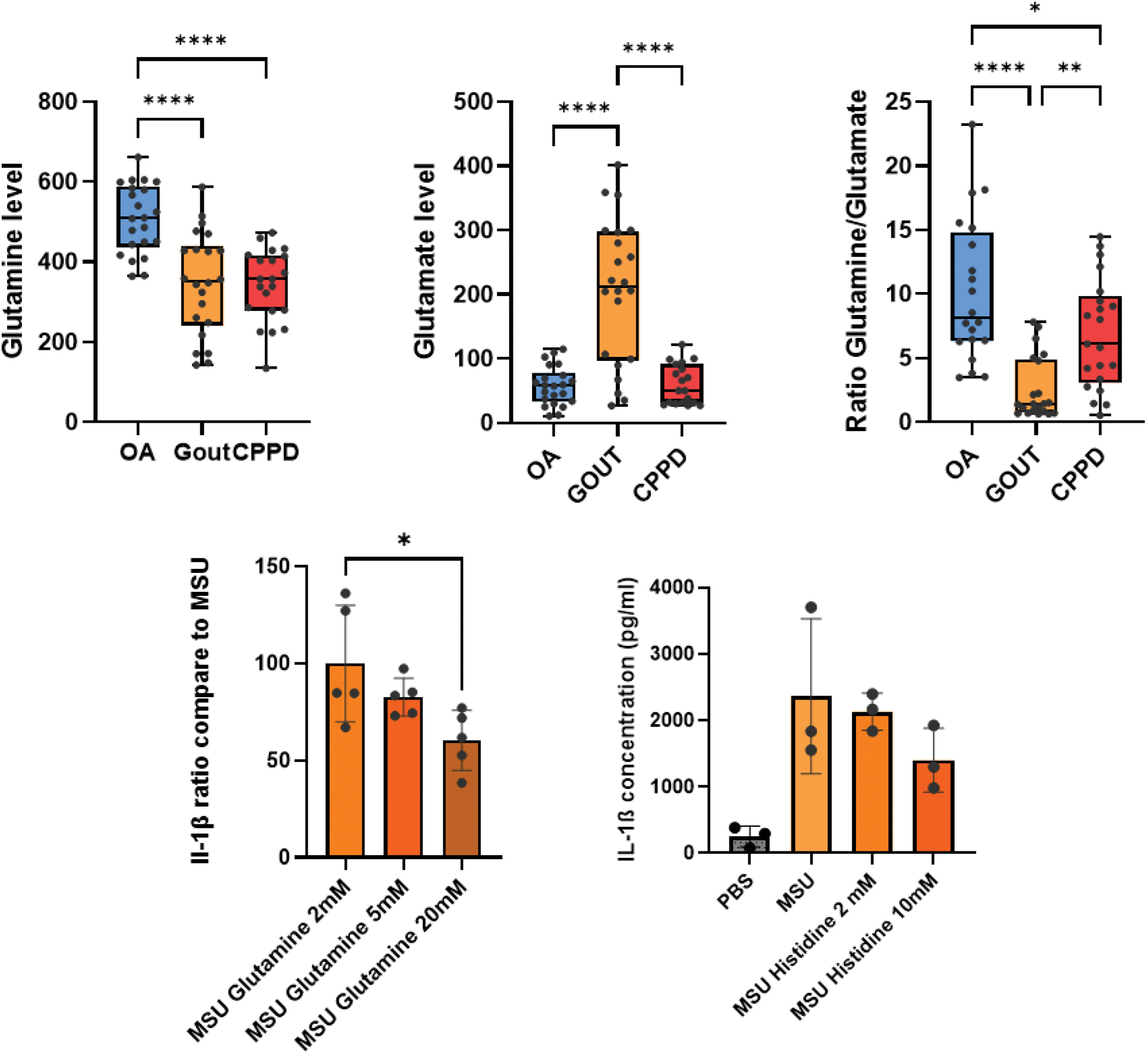
Results: Plasmas analysis by NMR identified 17 metabolites with different abondance during gout flare (T1) and flare resolution (T2): 6 metabolites (Glyc, GlycA, GlycB, Glyc/SPC, H2FC, Acetoacetic acid) were increased during the flare and 11 decreased (Histidine, H4TG, Glycine, Alanine, H4PL, H4A1, H4A2, Methionine, Glutamine, Lysine, H4CH) (Figure 1A). Correlation analysis showed a strong association between these metabolites and CRP. Especially, glutamine and histidine abondance was negatively correlated with CRP, TNFSF14 and IL-6 (Figure 1B), hinting for a potential role in inflammation regulation. Synovial fluid dosage of glutamine and glutamate level revealed that patients suffering from crystal driven inflammation had lower glutamine/glutamate ratios compared to mechanical osteoarthritis (OA) synovial fluid (Figure 2A-C). In vitro, supplementation of THP1 cells with 20mM of glutamine significantly reduced IL-1β concentration after MSU stimulation (Figure 2D). Histidine supplementation shows a tendency to decrease IL-1β concentration after MSU stimulation (Figure 2E).
Conclusion: Among the 17 metabolites associated with gout flare, the abondance of glutamine and histidine was diminished during the inflammation phase. These 2 metabolites possessed anti-inflammatory properties. Further studies are ongoing to identify how these metabolites reduce IL-1β production.
REFERENCES: NIL.
A) Volcano plot comparing metabolites levels during and after the flares. B) Histidine, Glutamine and known inflammatory cytokines levels adjusted to the CRP level during the flare.
A) B) C) Glutamine and glutamate levels/ratio in synovial fluid of joints affected by OA, Gout or CPPD. D)E) Il-1β concentration ratio of thp1 cells supplemented by glutamine or histidine and stimulated by MSU.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (