

Background: Several reports confirm the existence of persistent, subclinical inflammation related to monosodium urate (MSU) crystals, both in gout and asymptomatic hyperuricemia (AH) with crystal deposition. However, the mechanism behind it is poorly understood.
Objectives: To analyze the articular inflammatory proteome associated with MSU crystal deposition in hyperuricemic patients with no clinically active inflammation.
Methods: Observational, cross-sectional study. 100 participants with either AH (hyperuricemia defined as serum urate ≥7mg/dl) [n=77] or intercritical gout (2015 ACR/EULAR criteria, hyperuricemia, and no treatment) [n=23] underwent clinical assessment, blood testing, and sonographic assessment of knees, ankles, 1MTPs, and 2MTPs. In the case of effusion, synovial fluid (SF) was obtained. Two observers analyzed samples simultaneously under the polarized microscope for microcrystals (MSU, calcium pyrophosphate –CPP) and leukocytes (using a Neubauer chamber). SF proteome was studied using Olink proteomic assessment (Target 96 Inflammation panel) in samples with enough volume after centrifugation and cell extraction. For these cases, paired serum proteome assessment using Olink technology was intended. Comparisons were performed according to microcrystal deposition, identifying differential expression using principal component analysis and volcano plots. Correlations between quantitative variables were tested using Spearman’s rho.
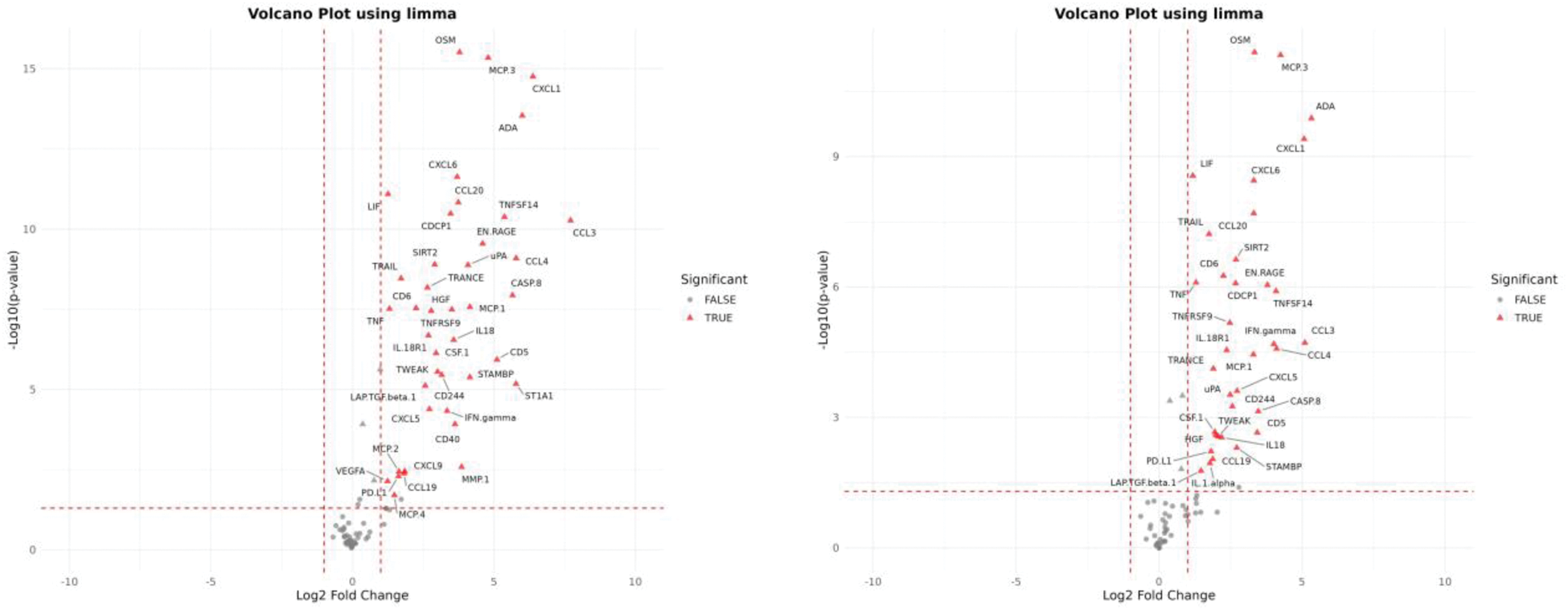
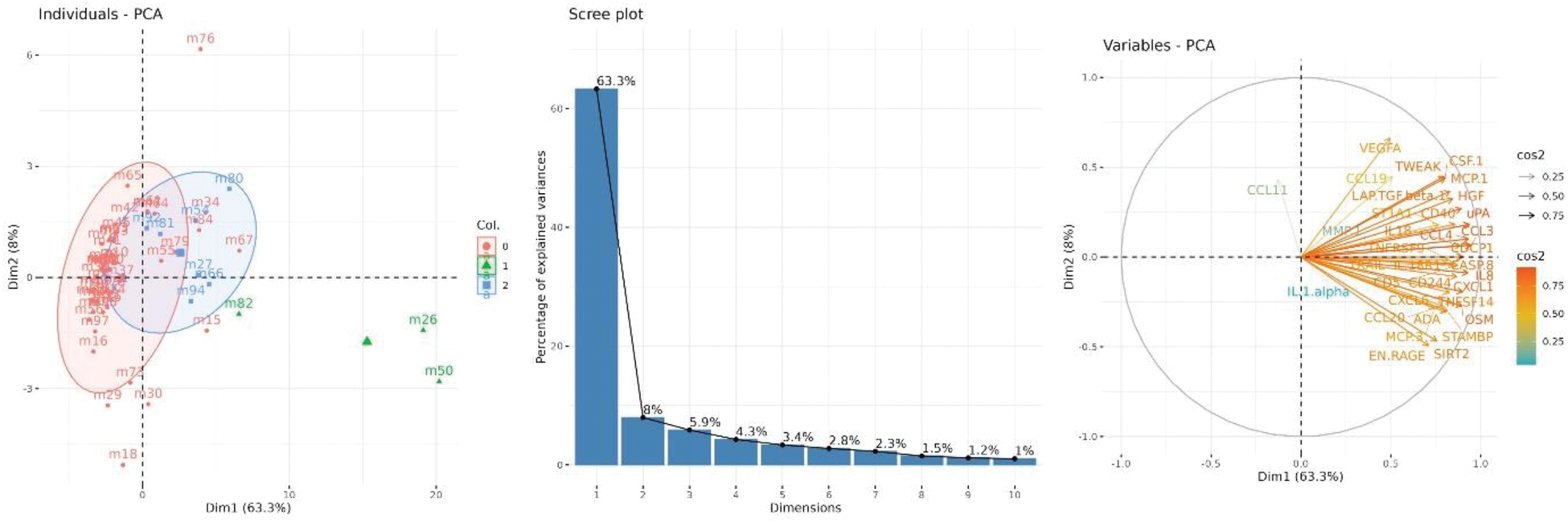
Results: Out of 100 participants studied, 81 had SF samples available for polarized microscopy; MSU and CPP crystals were detected in 5 (6.2%) and 11 samples (13.6%), respectively, while the others showed no crystals (65, 80.2%). Median leukocyte count was 50/mm3 (p25-75 20-90), being significantly higher in samples with MSU (640/mm3, 245-875) compared to CPP (50/mm3, 40-90) or no crystals (40/mm3, 20-70), p<0.001. SF leukocytes showed no correlation to serum levels of total leukocytes, urate, or C-reactive protein. 60 samples were available for proteomic analysis (3 with MSU, 8 with CPP, 49 with no crystals). Figure 1 shows the volcano plots comparing MSU versus CPP (left) and versus no crystals (right); both comparisons yielded similar results. SF containing MSU crystals showed an increased relative expression of adenosine deaminase (ADA), oncostatin M (OSM), chemokine ligand 1 (CXCL1), monocyte chemotactic protein 3 (MCP-3), granulocyte chemoattractant protein-2 (CXCL6), TNF superfamily protein 14 (TNFSF14), interleukin-8 (IL8), and chemokine ligand 3 (CCL3), among others. These proteins mostly contributed to differentiate samples among the three groups (71.3% of explained variances [Figure 2]). Only IL-8 showed a significant correlation between synovial fluid and serum expression (r=0.44, p=0.019) using paired sera (available for n=28).
Conclusion: Subclinical inflammation associated with MSU crystal deposition comprises raised leukocyte counts and a distinctive proteomic profile. Some inflammatory proteins (such as OSM and TNFSF14) had been previously found in sera of patients with flares [PMID 38373842]. However, during asymptomatic stages, the inflammatory process appears to be local and confined to the joint, the relevance of which needs to be further explained.
Volcano plot of differentially expressed proteins in SF samples (to the right favors MSU; to the left favors no crystals [left] or CPP [right]).
Left, principal component analysis of synovial fluid inflammatory proteins showing the distribution of patients’ samples across dimensions 1 (dim1) and dim2 [red=no crystals; green=MSU; blue=CPP]. Middle, percentages of explained variances by dimensions. Right, proteins distributions to dim1 and dim2 and their contribution.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Mariano Andrés Menarini, SOBI, Grunenthal, María-Luisa Peral-Garrido: None declared, Samanta Ortuño: None declared, Rocío Caño: None declared, Silvia Gómez: None declared, Alejandra Bermúdez-García: None declared, Teresa Lozano: None declared, Miguel Perdiguero: None declared, Elena Caro-Martínez: None declared, Ruth Sanchez-Ortiga: None declared, Carolina Ruiz-García: None declared, Rubén Francés: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (