

Background: Gout, due to the presence of monosodium urate crystals (MSU) and calcium pyrophosphate (CPP) crystal deposition disease are both responsible for recurrent inflammation flares. Both crystals stimulate interleukin-1β production by macrophages. Macrophages can interact and phagocytose both MSU and CPP crystals. In vivo, Giant multinucleated cells (GMC) are observed surrounding the MSU deposition in tophi from gouty patient.
Objectives: Describe the intracellular fates of MSU and CPP crystals in macrophages.
Methods: Synthetic pyrogen-free MSU and CPP crystals were used to stimulate different type of mouse (RAW 264.7 cell line, bone marrow-derived macrophages (BMDM)) and human (THP-1 cell line and peripheral blood mononuclear cells (PBMC)) macrophages. To assess crystal phagocytosis in vitro, macrophages were stimulated by MSU and CPP crystals engrafted with fluorescent organic nanoparticles (FON) and recorded for 5 days using the Incucyte instrument. Intracellular outcomes were assessed by 18-hours recording using apotome microscopy. Images analysis was performed by ImageJ software. Phagocytosis and cell to cell exchanges were observed by transmission electron microscopy using Raw cells after 3h and 60h of MSU stimulation respectively. To observe crystal phagocytosis by GMCs ex vivo, tophi from gouty patient were fixed and stained by Von Kossa and HE staining. In vitro GMC were obtained after stimulation of RAW 264.7 cells by MSU, after complete dissolution of crystals and macrophages fusion to form GMC at day 10, GMC were re-stimulated by MSU and recorded.
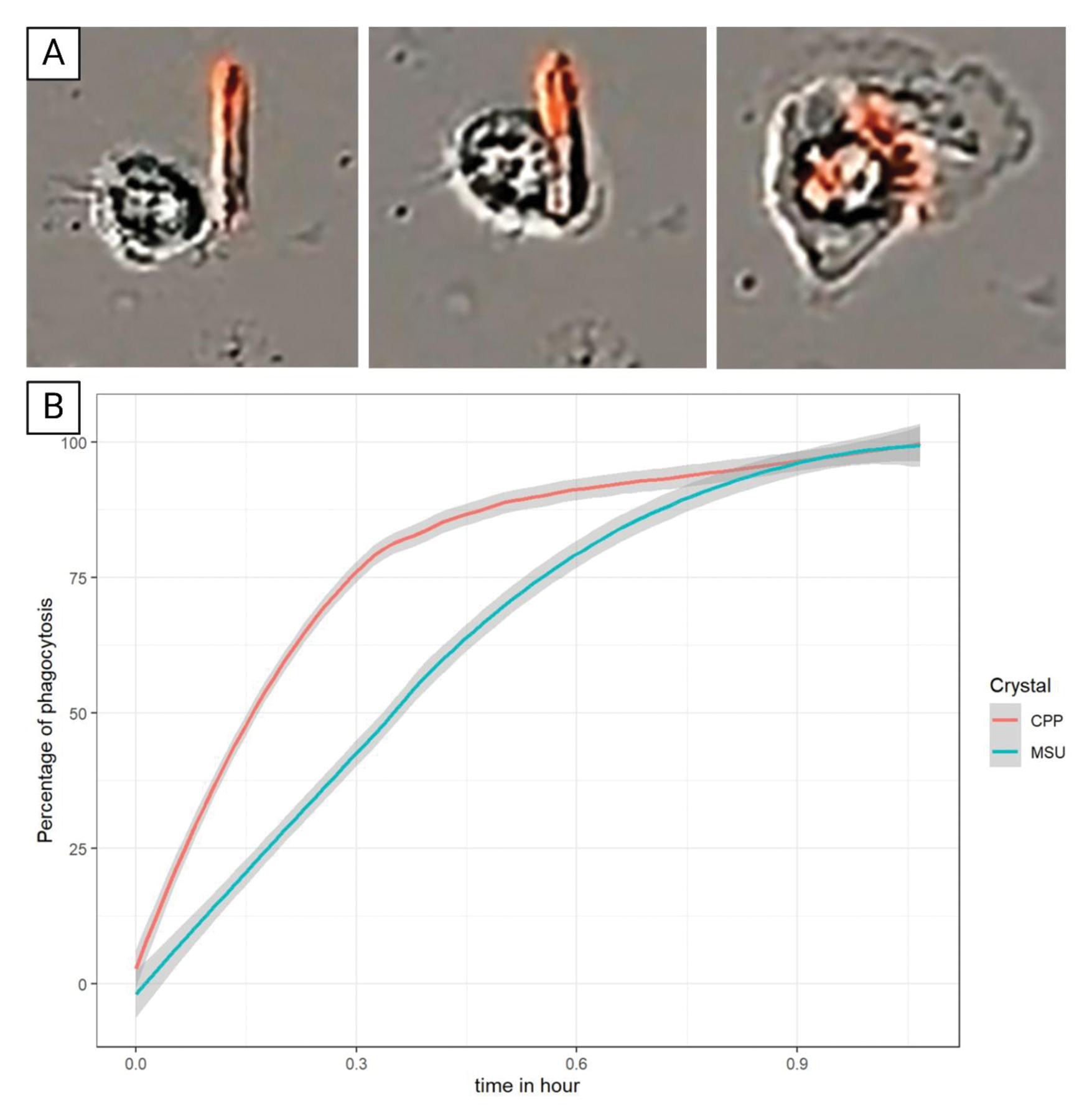
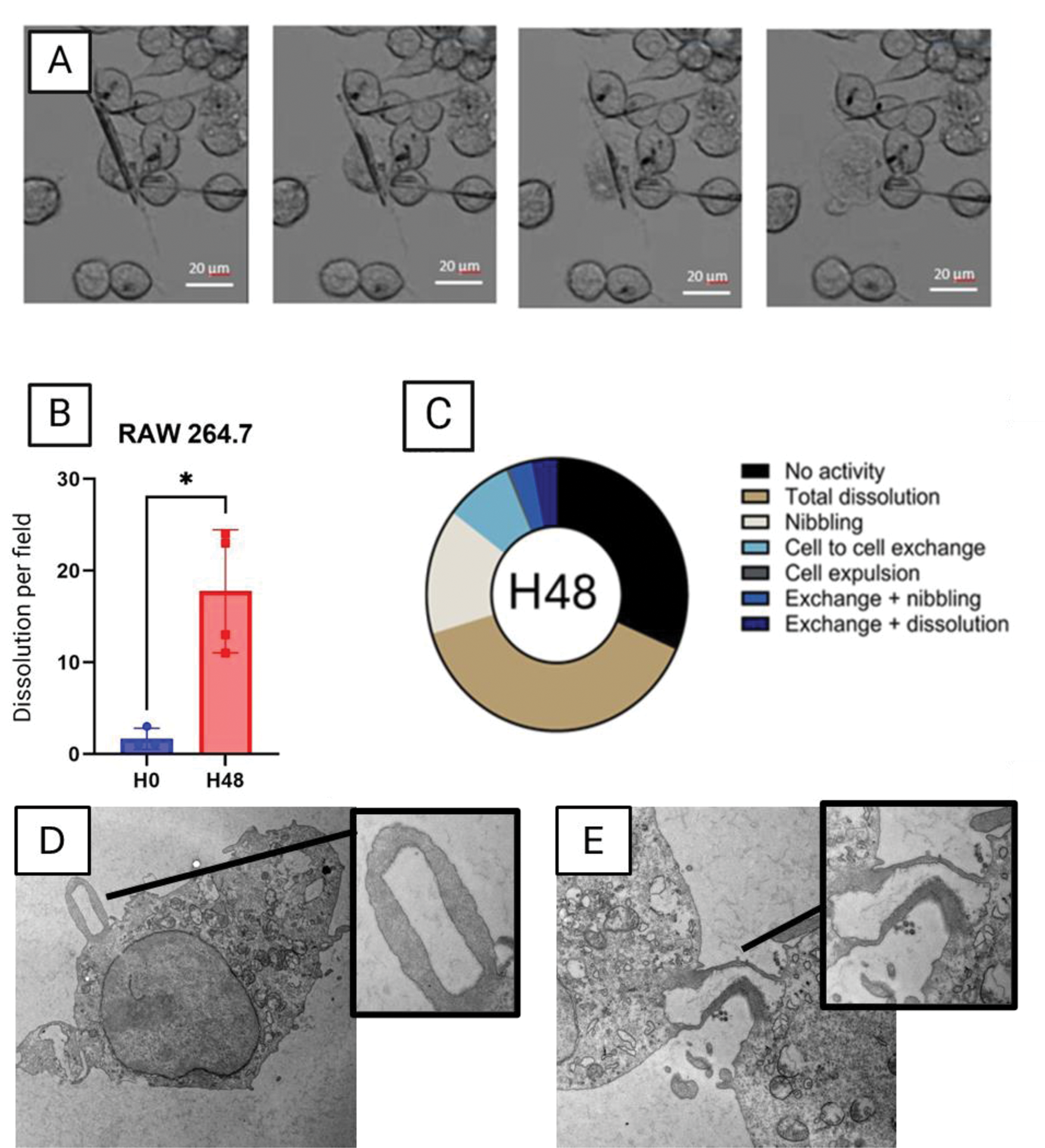
Results: All type of macrophages including RAW 264.7, BMDM, THP-1 and PBMC, phagocytosed MSU and CPP crystals within one hour (Figure 1). MSU crystals were dissolved after phagocytosis while CPP crystals remained undissolved even after 7 days of stimulation. MSU crystals dissolution occurred only 48 hours after phagocytosis. No dissolution was observed in the first 48 hours while all MSU crystals were dissolved after 7 days of stimulation. Macrophages displayed different capacity to dissolve MSU crystals: after 48 hours of stimulation, 17.5% of MSU crystals (in one recording field) were dissolved in RAW 264.7 vs 24.3% and 56.0% for BMDM and THP-1, respectively (Figure 2A, B). Interestingly, we observed that dissolution of MSU crystals was followed by cell death and burst in RAW 264.7 and PBMC. THP-1 also dissolved MSU crystals but without burst nor death. Interestingly, internalized MSU crystals had various intracellular fates (Figure 2C). Crystals can be exchanged from one cell to the other (8.3%) or be partially dissolved at several areas of the crystal (15%).Phagocytosis and cell to cell exchanges were confirmed by TEM (Figure 2D,C) Treatment with the inhibitor of the V-ATPase pump, bafilomycin A1, completely blocked the dissolution of MSU crystals without affecting crystal phagocytosis Ex vivo, tophi analysis evidenced that GMC surrounding MSU crystal deposition had numerous intracellular MSU crystals, suggesting that they had been phagocytosed. Under MSU crystal stimulation these macrophages differentiated into GMC, which phagocytosed and dissolved numerous MSU crystals.
Conclusion: Our results suggest that dissolution of MSU crystals is a late cellular response involving cell lysosomes and acidification. Internalization of the crystals can lead to different intracellular fates. GMC seems to present a higher capacity to phagocyte and dissolve MSU crystals in vitro. Furthers studies are ongoing to decipher the underlying mechanisms.
REFERENCES: NIL.
A) Phagocytosis of FON coupled CPP by PBMC during the first hour. B) Percentage of phagocytosis of MSU and CPP crystals by PBMC
A) Dissolution of MSU crystal by RAW 264.7 cells accompanied by the bursting and death of the cell after 48h of stimulation. B) quantification of the number of dissolutions of MSU crystals per field of three type of cells. C) Various intracellular fate of MSU crystals after 48h of stimulation in three types of cells. D) MSU phagocytosis by Raw 264.7 cells observed in TEM after 3h of stimulation. E) Exchange of MSU crystal from one cell to the other observed in TEM after 60h of stimulation
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (