

Background: Recent studies have demonstrated that the formation of neutrophil extracellular traps (NETs) exerts a bidirectional regulatory influence on gout-related diseases. NETs not only contribute to the induction and amplification of inflammation but also play a crucial role in resolving inflammatory responses. Notably, peptidylarginine deiminase 4 (PAD4), an essential immune mediator, is pivotal in the formation of NETs.
Objectives: This study utilized single-cell transcriptome sequencing technology to investigate the heterogeneity of peripheral blood neutrophils in gout patients, hyperuricemia patients, and healthy controls, identifying the differential gene PADI4. Subsequently, a gout mouse model was established to examine the therapeutic efficacy and underlying mechanisms of targeting Peptidyl arginine deaminase 4 (PAD4) in inhibiting Neutrophil extracellular trap (NET) formation in gout mice. This research provides novel insights into the pathogenesis and treatment strategies for gout.
Methods: Part I: (1) Fresh peripheral blood neutrophils were isolated from patients with gout (n=9), individuals with hyperuricemia (n=6), and healthy controls (n=4). Single-cell transcriptome sequencing was subsequently performed on these samples. (2) The formation rates of neutrophil extracellular traps (NETs) induced by monosodium urate (MSU) crystals at concentrations of 125 μg/ml, 250 μg/ml, and 500 μg/ml were compared in peripheral blood neutrophils. Part II: Experimental Study on the Treatment of Gout via Targeted Inhibition of PAD4 to Suppress NETs Formation (1) A gout mouse model was successfully established. Following the confirmation of successful modeling, PAD4 was specifically targeted and inhibited for therapeutic intervention, with subsequent monitoring of changes in the diameter of the mouse ankle joints. (2) Forty-eight hours post-modeling, the mice were euthanized to facilitate the examination of pathological changes in their joints. Immunofluorescence analysis was performed to identify and label NETs within the mouse ankle joints. (3) The expression levels of PAD4 mRNA and inflammatory factors in the mouse ankle joints were quantified using RT-PCR.
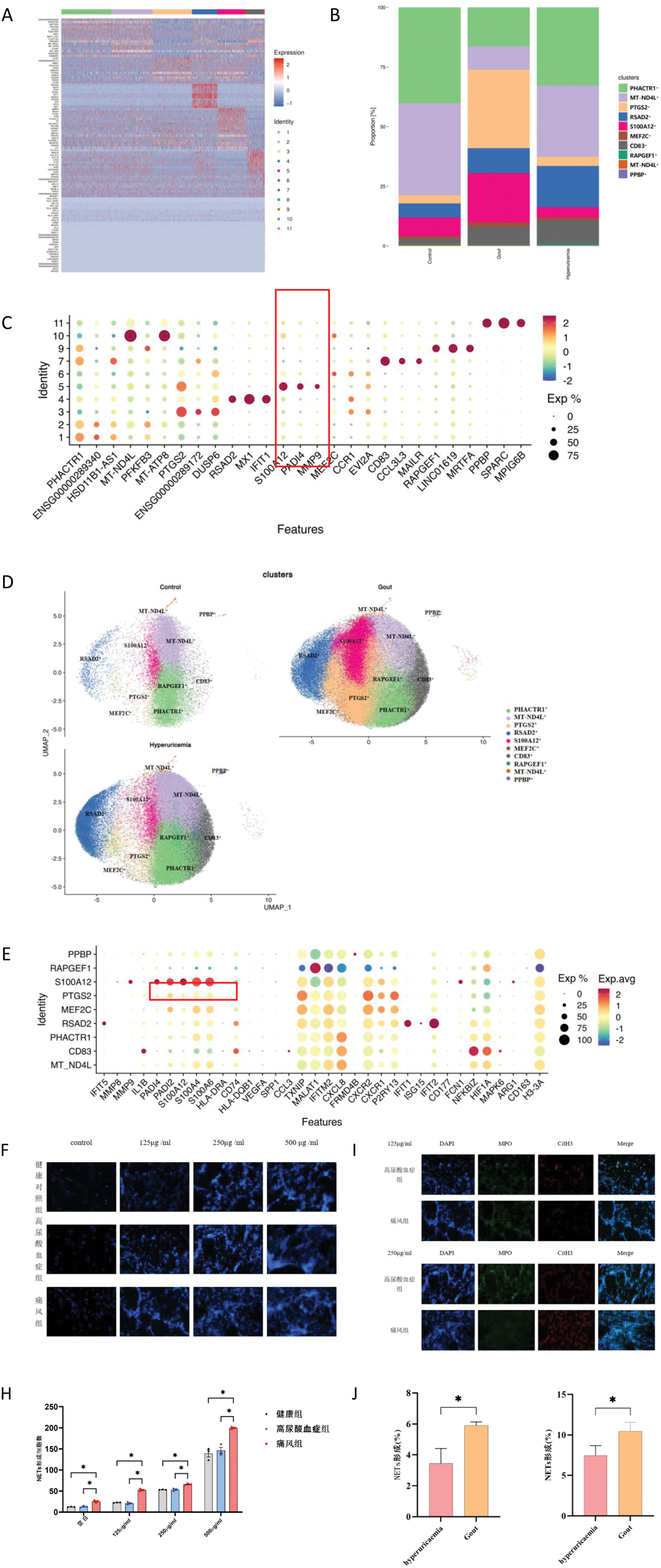
Results: The initial section: Neutrophils from diverse populations demonstrate heterogeneity and exhibit varying sensitivities to MSU crystal stimulation. 1) Single-cell transcriptome sequencing was conducted on the samples, and cell subpopulations were systematically annotated. It was observed that the S100A12+neu subpopulation, which exhibited a relatively higher cell count in gout patients, harbored the target gene PADI4 among its marker genes (Figure 1A-E). 2) Various concentrations of MSU crystals can induce NET formation, with the number of NETs increasing in a dose-dependent manner (Figure 1F-H). At identical concentrations of MSU crystals, the gout group exhibited a significantly higher production of NETs compared to the asymptomatic hyperuricemia group (Figure 1I-J).
Analysis of Neutrophil Heterogeneity. A-E. Nomenclature and classification of cell subgroups along with their respective marker genes; F-J. Differential responses of three distinct groups of individuals to varying concentration gradients of MSU suspension.
Part Two: The targeted inhibition of PAD4 can diminish the production of NETs, thereby alleviating joint inflammation. (1) At 0, 12, 24, and 48 hours post-modeling, the extent of ankle swelling in mice was evaluated. The gout model group exhibited significant ankle swelling. Targeted inhibition of PAD4 resulted in a notable reduction in joint swelling compared to the model group (Figure 2A-C). (2) Histopathological analysis revealed that the gout model group had the most pronounced inflammatory cell infiltration in the ankle joint. Following targeted inhibition of PAD4, these changes were significantly alleviated compared to the model group, with statistically significant differences observed (Figure 2D-E). (3) Treatment with GSK484 and siRNA led to a significant reduction in the expression levels of PAD4 and pro-inflammatory factors, as evidenced by statistically significant differences (Figure 2F-I). (4) Immunofluorescence analysis demonstrated the presence of typical NET structures in the ankle joints of the model group, with a higher formation rate compared to the PAD4-targeted inhibition group. Targeted inhibition of PAD4 effectively reduced NET production (Figure 2J-M).
Targeted Inhibition of PAD4 Attenuates Joint Inflammation. A-C: Impact of PAD4-targeted inhibition on joint swelling in gout-induced mice. D-E: Hematoxylin and Eosin (HE) and Tartrate-Resistant Acid Phosphatase (TRAP) staining of ankle joints from mice. F-I: Expression levels of PAD4 mRNA and associated inflammatory markers. J-M: Effects of PAD4-targeted inhibition on Neutrophil Extracellular Trap (NET) formation.
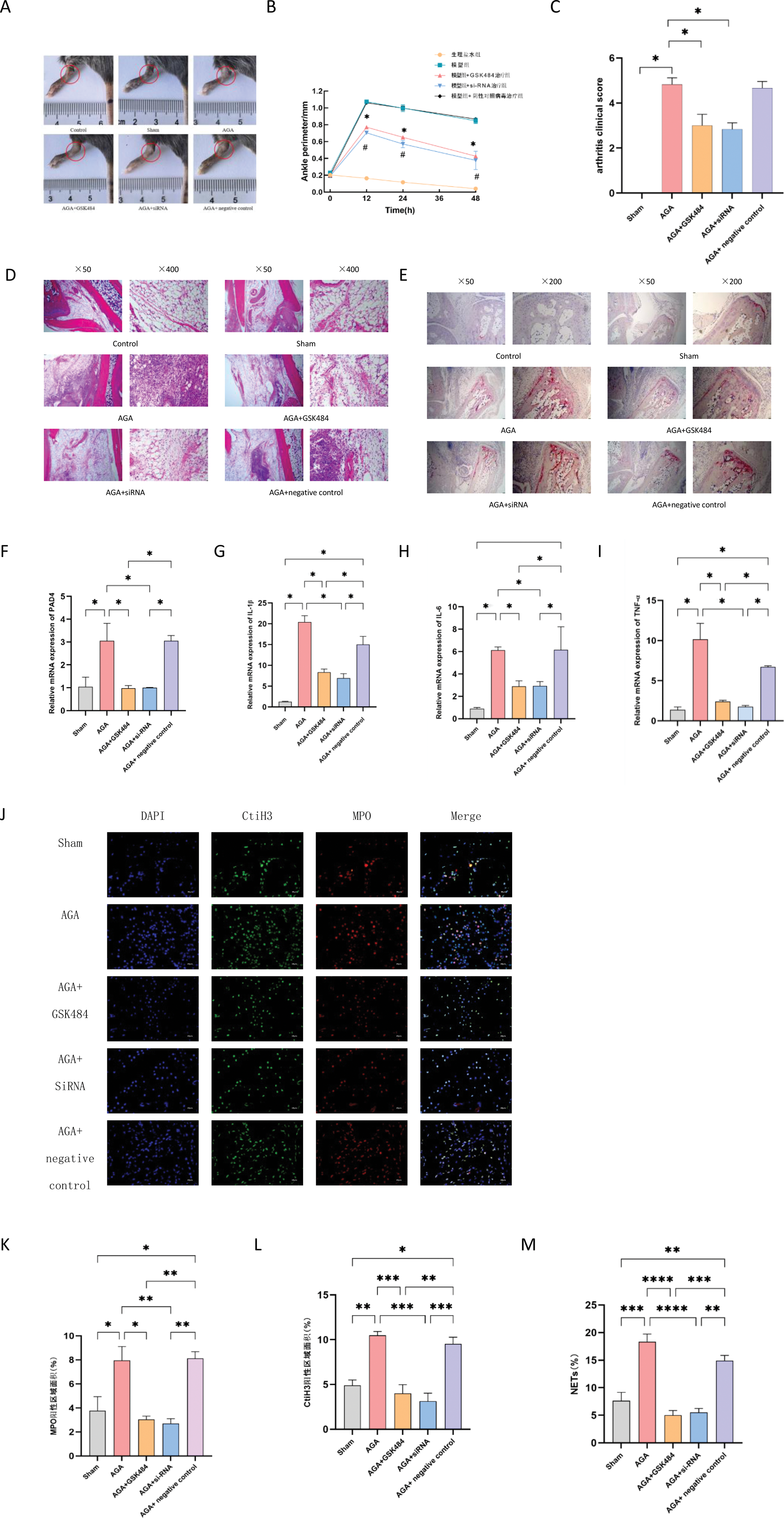
Conclusion: The differential gene expression profiles of peripheral blood neutrophils were initially constructed for both gout patients and asymptomatic hyperuricemia patients. A notable subpopulation, S100A12+ neutrophils, which is relatively abundant in gout patients, was identified. The target gene PADI4 was included among its marker genes. PAD4 plays a crucial role in the formation of neutrophil extracellular traps (NETs) and further contributes to the pathogenesis of arthritis in gout model mice. Silencing PAD4 mRNA via RNA interference (RNAi) and inhibiting PAD4 expression using the PAD4 inhibitor GSK484 have been shown to alleviate joint inflammation in mice, reduce inflammatory factors, and achieve therapeutic effects.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (