

Background: Hydrogen sulfide (H 2 S) is a gasotransmitter involved in various pathophysiological processes, including inflammation. Physiological and pharmacological levels of H 2 S can be reached, both in vitro and in vivo , using H 2 S donors. H 2 S is produced in cells by three enzymes, one of which is cystathionine gamma lyase (CSE). NLRP3 inflammasome is a multiprotein complex that can be activated by monosodium urate (MSU) crystals, the etiological factor in gout. When the inflammasome is activated, ASC oligomerizes, leading to the activation of caspase-1, which cleaves pro-IL-1β into its active, secreted form. We previously demonstrated that both exogenous and endogenous H 2 S inhibit NLRP3 inflammasome activation in macrophages in vitro and in murine peritoneal fluids in vivo [1].
Objectives: To test if positive allosteric activators of CSE (CSE-PAMs) could inhibit NLRP3 inflammasome activation by MSU crystals.
Methods: A CSE-PAM (SAN523) was identified and tested for its ability to enhance H₂S production by recombinant or cellular CSE. H 2 S production was measured using the H 2 S specific fluorescent probe AzMc or the lead acetate method. Supernatants from primed murine bone-marrow derived macrophages (BMDM) and human macrophage-like THP1 cells, stimulated with 500 µg/ml MSU crystals for 6h, in the presence or absence of SAN523 (0.1-50 µM) were analyzed by IL-1β Western-Blot (WB) and ELISA. In vivo , peritonitis was induced in mice by i.p. injection of 1 mg MSU crystals, with SAN523 treatment (5 mg/kg) or vehicle (DMSO) administered i.v. 15 minutes prior to MSU injection. THP1 cells, stimulated with 500 µg/ml MSU crystals for 1h, in the presence or absence of SAN523 (50 µM). Lysates were analyzed by the maleimide assay for detection of protein persulfidation.
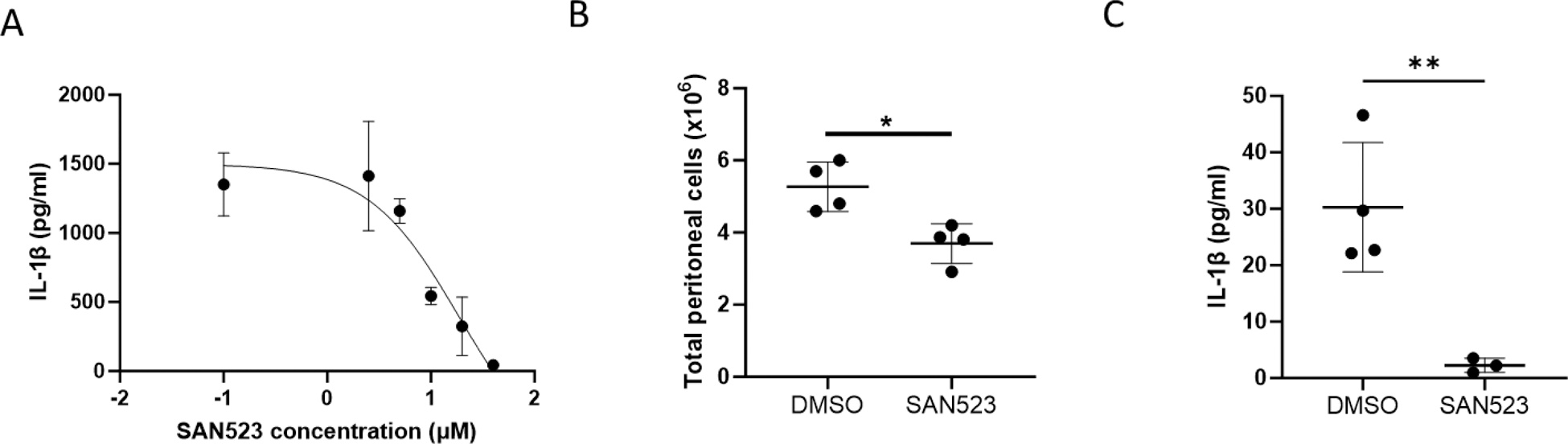
Results: SAN523 stimulated in a dose-dependent way H 2 S production by human recombinant CSE and by THP1 cells. In addition, SAN-523 reduced ASC oligomerization (% ASC speck positive cells over total cells: MSU = 11.5; MSU+SAN523 = 3.5, p = 0.037) and inhibited in a dose-dependent way IL-1β secretion in primed THP1 macrophages stimulated with MSU crystals (IC50 = 1µM, Figure 1A). A similar decrease in IL-1β secretion was observed in primed BMDMs. Inhibition of IL-1β secretion by SAN523, in both human and murine cell supernatants, was confirmed by WB. In the MSU-induced peritonitis model, pretreatment with SAN523 significantly reduced inflammatory cell recruitment (millions of cells: MSU = 5.3; MSU+SAN523 = 3.7, p = 0.012, Figure 1B) and IL-1β levels in peritoneal fluid (IL-1β in pg/ml: MSU = 30.27; MSU+SAN523 = 2.27, p = 0.009, Figure 1C), as well as other inflammatory cytokines and chemokines such as IL-6, IL-1α, Cxcl1. Finally, we identified that SAN523 acts, at least partly, via induction of protein persulfidation.
A) Secretion of IL-1β (pg/ml) by THP1 cells determined by ELISA; experiment performed in triplicates. B) Inflammatory cell recruitment in murine peritoneal lavage n=4. C) Secretion of IL-1β (pg/ml) in murine peritoneal lavage n=4.
Conclusion: SAN523 enhances H 2 S production leading to reduced NLRP3 inflammasome activation in vitro and in the MSU-induced peritonitis model in vivo . This new compound can be of interest as a novel drug for acute gout and other IL-1β mediated inflammatory diseases.
REFERENCES: [1] M. Castelblanco, J. Lugrin, D. Ehirchiou, S. Nasi, I. Ishii, A. So, F. Martinon, N. Busso, Hydrogen sulfide inhibits NLRP3 inflammasome activation and reduces cytokine production both in vitro and in a mouse model of inflammation, J Biol Chem 293(7) (2018) 2546-2557.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (