

Background: Modic type 1 changes (MC1) are painful vertebral bone marrow lesions adjacent to degenerated intervertebral discs. Bone marrow inflammation is a hallmark of MC1 lesions, but a comprehensive understanding of the immune system’s role in them is missing.
Objectives: To investigate the immunological background of MC1 in relation to degeneration of the adjacent intervertebral disc.
Methods: Vertebral bone marrow aspirates were collected from cLBP patients with MC1 (n=22, MC1 +i ntra-patient control=8 + 8, control patients=6) undergoing lumbar spinal fusion, mononuclear cells were isolated and investigated by massively parallel flow cytometry. T-cell functional properties were analysed with flow cytometry: 1.) ex vivo analysis of Granzyme B, 2.) cytokine release after in vitro stimulation with PMA/ionomycin, and 3.) proliferation in response to IL-15 followed with CFSE labelling. Single-cell RNA-sequencing (scRNA-seq) was performed on enzymatically digested, neutrophils-depleted MC1 and intra-patient control biopsies (n=4 + 4). Extracellular matrix (ECM) proteins of intervertebral discs were analysed by LC-MS/MS. Cartilage oligomeric matrix protein (COMP) was quantified in bone marrow plasma with ELISA. Finally, bone marrow-derived mononuclear cells were treated with COMP, and cytokine production was analysed using flow cytometry.
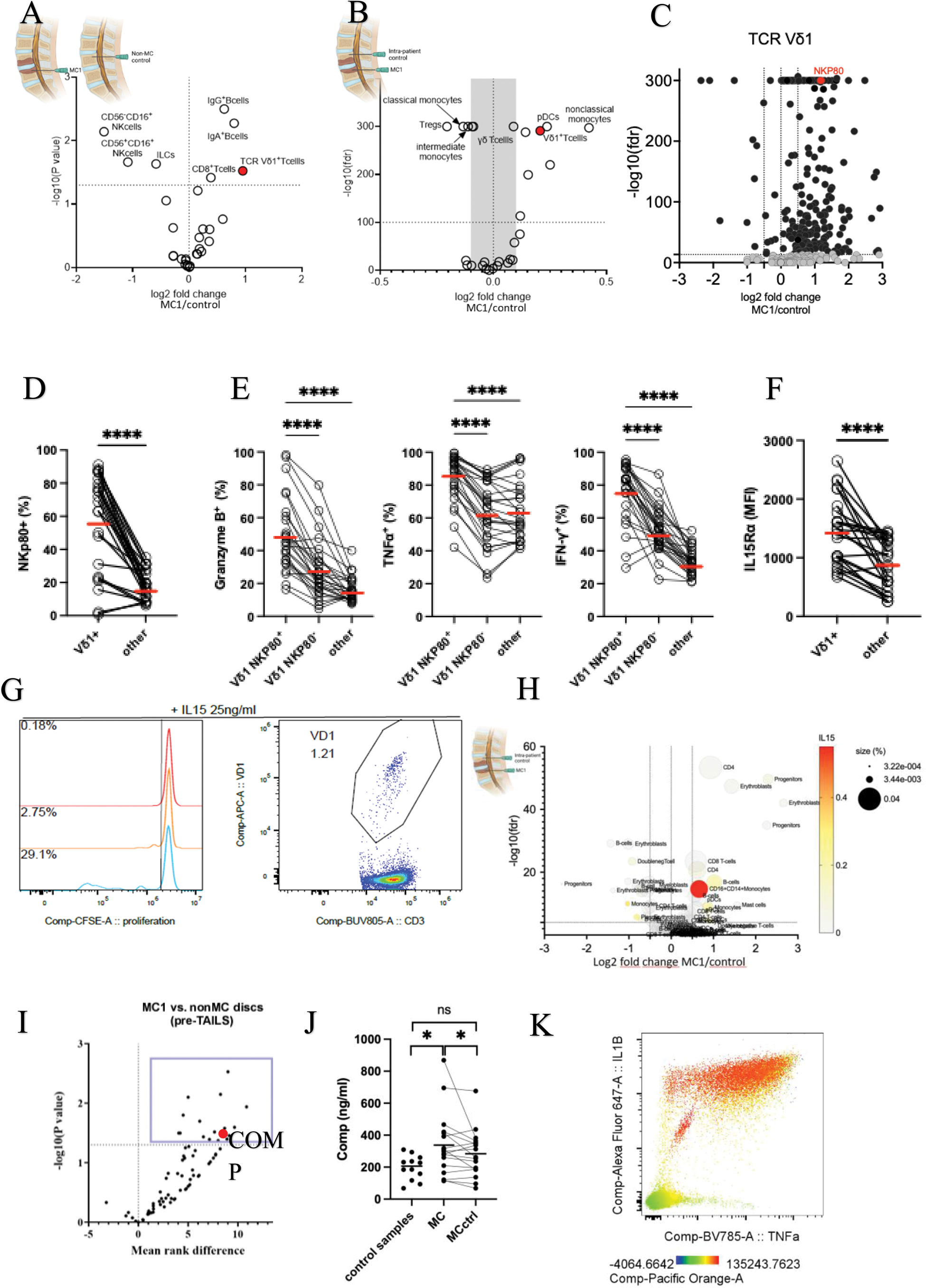
Results: Immunophenotyping revealed accumulation of Vδ1 T-cells in MC1 (Figure 1A,B) and significant differences in the expression of surface markers on Vδ1 T-cells in MC1 (Figure 1C). Most Vδ1 T-cells in MC1 expressed NKp80 (72% vs. 40% in intra-patient control), and NKp80 was more expressed on Vδ1 T-cells than on other T-cells (Figure 1D). NKp80 + Vδ1 cells expressed more Granzyme B and produced more IFNγ and TNF upon stimulation than other T-cells (Figure 1E). Moreover, Vδ1 T-cells expressed higher levels of IL15Rα than other T-cells (Figure 1F), and IL-15 selectively induced their proliferation (Figure 1G). Bone marrow plasma IL-15 levels correlated with Vδ1 frequency (R=0.84, P<0.001). ScRNAseq revealed that IL-15 was predominantly expressed by CD16 + monocytes and that CD16 + monocytes accumulated in MC1 (Figure 1H). ECM component COMP was enriched in intervertebral discs adjacent to MC1 and in MC1 bone marrow (Figure 1I,J,K) and COMP activates monocytes in vitro (K).
A .) Comparison of immune cell subset presence in MC1 patients and patients without MC1 based on flow cytometry screening of bone marrow aspirates. B .) Comparison of immune cell subset frequencies in MC1 lesions and intrapatient controls based on flow cytometry screening of bone marrow aspirates. C .) Changes in the expression of surface markers on T-cells from MC1 and control tissue. Markers mentioned in the text are highlighted in red. D. ) Comparison of NKp80 expression between T cell subsets. E.) Comparison of Granzyme B, IFNγ, and TNFα expression between NKp80 + Vδ1 T-cells and other T-cells. F .) Comparison of CD215 expression between T cell subsets. MFI=Median Fluorescence Intensity G. ) The proliferation of CD4 + αβ T-cells, CD8 + αβ T-cells, and Vδ1 T-cells in response to IL15. H. ) Comparison of clusters of immune cell presence in MC1 lesions and intrapatient control based on single-cell RNA seq of bone marrow biopsies. Expression of IL15 RNA is shown as color code. I .) Comparison of protein presence in intervertebral discs of MC1 and control patients using mass spectrometry. J .) Comparison of COMP levels in bone marrow plasma in MC1 lesions, intrapatient controls, and control patients by ELISA. K. ) Activation of PBMCs by 20ug/ml of COMP. Expression of TNFα, IL1β, and IL14 was followed by flow cytometry. The expression of CD14 is depicted as a color code.
Conclusion: We discovered an accumulation of cytotoxic and pro-inflammatory Vδ1 T-cells in MC1 lesions that might be linked to degeneration of the adjacent intervertebral disc. Their proliferation was closely related to bone marrow levels of IL-15, which also induces their proliferation in vitro . A subset of CD16 + monocytes accumulating in MC1 was discovered as the primary source of IL-15 in MC1, and we revealed that they can be activated by ECM protein COMP, which also accumulates in MC1. Our findings thus suggest direct link between ECM degradation and the activation of immune system in MC1 positioning ECM degradation products to the role of potential treatment targets.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Jan Devan Novartis, Swiss Life, Irina Heggli: None declared, Michaela Sandalova: None declared, Dominik Burri: None declared, Pamela Bitterli: None declared, Nick Herger: None declared, Danilo Menghini: None declared, Phelipe Luan Hatt: None declared, Kenta Robin Brender: None declared, Melina Perez Vertti Valdes: None declared, Christoph Laux: None declared, Mazda Farshad: None declared, Oliver Distler consultancy relationships with and/or has served as a speaker for the following companies in the area of potential treatments for systemic sclerosis and its complications in the last three calendar years: 4P-Pharma, Abbvie, Acceleron, Acepodia Biotech, Aera, Alcimed, Altavant, Amgen, AnaMar, Anaveon AG, Argenx, AstraZeneca, Blade, Bayer, Boehringer Ingelheim, Calluna (Arxx), Cantargia AB, Catalyze Capital, Corbus, CSL Behring, Galderma, Galapagos, Glenmark, Gossamer, Horizon, Janssen, Kymera, Lupin, Medscape, MSD Merck, Miltenyi Biotec, Mitsubishi Tanabe, Nkarta Inc., Novartis, Orion, Pilan, Prometheus, Quell, Redxpharma, Roivant, EMD Serono, Topadur and UCB. Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143). Co-founder of CITUS AG. Research Grants: BI, Kymera, Mitsubishi Tanabe, UCB, Stefan Dudli: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (