

Background: Autoimmune Inflammatory Myositis (AIM) can be divided into distinct subsets, including dermatomyositis (DM), overlap myositis (OM), immune-mediated necrotizing myositis (IMNM), and inclusion body myositis (IBM), some being associated with antibodies. However, despite this putative link with the presence of autoantibodies, B cell contribution to disease pathophysiology and their role in disease severity and chronicity remains unknown. We therefore reported on 8 patients with AIM that were not DM but surprisingly exhibited B-cell infiltration with prominent B cell aggregates in muscle biopsies [1]. B-cell infiltration in muscle tissue from non-DM AIM is a novel concept and its implication remains uncertain. This raises the crucial question of whether patients displaying B cell aggregates (BCM) at biopsy are more likely to benefit from treatment with agents targeting specific B cells subsets (e.g., rituximab for CD20+ B cells or daratumumab for CD38+ plasma cells). A thorough characterization of the B cells enriched AIM is thus essential to inform the initiation future well-justified clinical studies.
Objectives: We aim to characterize the inflammatory infiltrate present in muscle biopsies of patient with BCM (focusing on B and T cells) and assess how they differ from myositis controls without B-cell aggregates, using cyclic immunofluorescence (Cyc-IF).
Methods: Muscle biopsies of 22 BCM subjects and 24 controls (AIM without prominent B-cell aggregates) in the Canadian Inflammatory Myopathy Study (CIMS) cohort and biobank were retrieved. B-cell aggregates were defined as >30 CD20+ cells/aggregate. Controls were further classified according to clinical, serological and histopathological profile into either DM, OM or IBM. The nature of the immune infiltrate was resolved by Cyc-IF. This staining method enables the spatial detection of up to >40 proteins on a single slide. Muscle biopsies were fixed in formalin and processed for paraffin embedding according to standard procedure of the pathology laboratory. Tissue sections of 5 um were used for Cyc-IF staining. Cyc-IF involves multiple sequential rounds of immunofluorescence staining, imaging, and quenching. Prior to staining, the slides are incubated in a blocking solution and autofluorescence level acquired (Axioscan 7). For each staining cycle, a set of 4 primary antibodies conjugated with either Alexa-Fluor 488, 555, 647 or 750 are incubated on the tissues. The slides are washed, the images acquired and then the slides are quenched in preparation for next round of staining. Overlayed Cyc-IF images are analyzed using a customized version of the Cyc-IF analysis python pipeline (github:
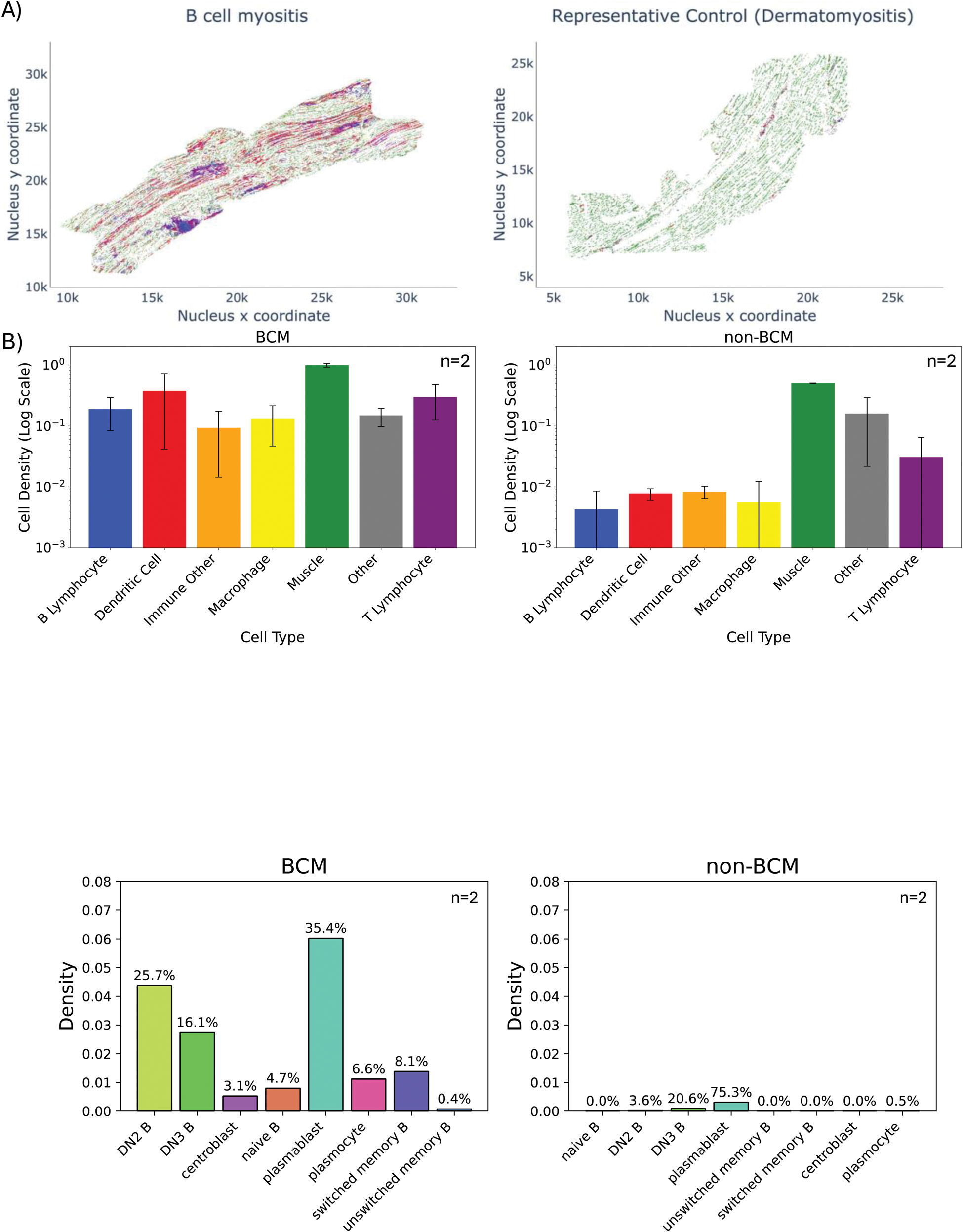
Results: Preliminary analysis was performed on 4 samples (2 BCM, 1 DM, 1 IBM). The infiltrates were initially characterized for major immune cell types and their respective densities. BCM biopsies had increased immune cell density compared to control (B cells, T cells, dendritic cells and macrophages). Digital reconstruction of the muscle tissue showed aggregates of B cells surrounded by T cells resembling tertiary lymphoid structures (TLSs; aggregate >100 CD20+ B cells). For further analysis, we excluded TLSs as we posit that they are uniformly constituted and, therefore, do not provide insight into the underlying mechanisms driving immune cell infiltration leading to TLS formation and their role in AIM pathophysiology. We aimed to further characterize the B cell subsets infiltrating the tissue. We specifically focused on examining naïve, memory, and tissue-infiltrating double-negative (IgD-/CD27-) B cells subsets, the implications of which have been reported in the pathophysiology of B cell-mediated diseases. B-cell infiltration from BCM patients revealed a predominance of plasmablasts, as well as DN3 (CD19 + /IgD-/CD27-/CD11c-/CXCR5-) and DN2 (CD19 + /IgD-/CD27-/CD11c+/CXCR5-). DN B cells are known to be the dominant infiltrating subsets in other diseases such Lupus and IgG4-RD. Other subsets detected in BCM samples included naïve B cells, switched and unswitched memory B cells. These findings support the hypothesis of the muscle acting like the host tissue for TLSs in AIM.
Conclusion: Our results suggest that BCM patients present a specific immunophenotype compared to inflammatory myopathies without B-cell aggregates, particularly in terms of the density of tissue infiltration by immune cells, mainly infiltrating B cell subsets. If confirmed across the rest of the cohort, these differences could provide insights on the immunological mechanisms underlying BCM and have the potential to help tailor future treatment of AIM patients with BCM.
REFERENCES: [1] Meyer A, Troyanov Y, Korathanakhun P, Landon-Cardinal O, Leclair V, Allard-Chamard H, et al. Myositis with prominent B cell aggregates may meet classification criteria for sporadic inclusion body myositis. Neuromuscular Disorders. 2023 Feb 1;33(2):169–82.
Acknowledgements: Myositis Canada.
Disclosure of Interests: Barath Ramanathan: None declared, Hao Chen Shen: None declared, Zoe Gerber: None declared, Celia del Carmen Crespo: None declared, Jason Karamchandani: None declared, Erin O’Ferrall: None declared, yves troyanov Astra Zeneca, Kezar Life Science, Eli Lilly, UCBs, Océane Landon-Cardinal: None declared, Josiane Bourré-Tessier: None declared, Marie Hudson Astra-Zeneca, Boehringer Ingelheim, Merck, Merck, Boehringer Ingelheim, Pfizer, Valérie Leclair: None declared, Marilyne Labrie: None declared, Hugues Allard-Chamard Abbvie, Amgen, Astrazeneca, BMS, Celltrion, Eli Lilly, Hoffmann-La Roche, Fresenius Kabi, GSK, Janssen, Novartis, Mantra Pharma, Pfizer, Sobi, Abbvie, Amgen, Astrazeneca, BMS, Celltrion, Eli Lilly, GSK, Hoffmann-La Roche, Janssen, Novartis, Pfizer, Sobi, Eli Lilly, Fresenius Kabi, Pfizer.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (