

Background: Idiopathic immune myopathies (IIMs) are a group of autoimmune diseases that produce chronic inflammation and degeneration of skeletal muscle structure and function. One process that maintains the integrity of muscle fibers is the plasma membrane repair process, which uses various cellular processes to reseal disruptions in sarcolemma and allow the cell to survive various insults. We have previously reported a sarcolemmal membrane repair defect in an IIM mouse model (AT mice) where RAG1-/- mice receive an adoptive transfer from SytVII-/-/FoxP3-/Y mice. One contributor to this repair defect was autoantibodies targeting TRIM72/MG53, an essential membrane repair protein, we found in the AT mouse sera and also in IIM patient sera. However, in that IIM patient population we identified additional sera samples with low levels of TRIM72/MG53 autoantibodies that still compromise repair capacity.
Objectives: Here we tested if autoantibodies against other proteins involved in membrane repair appear in IIM patients and if these could also compromise membrane repair.
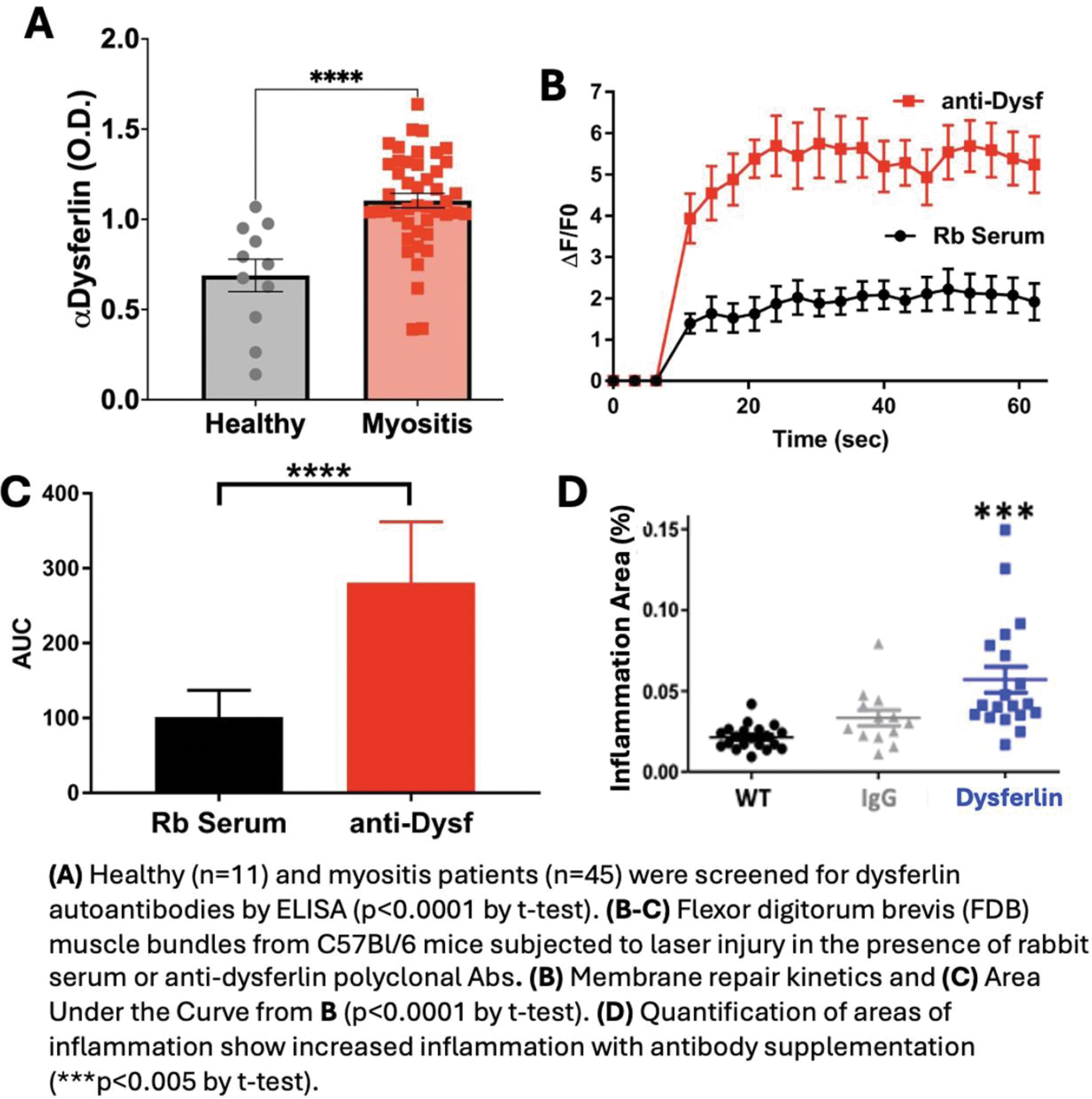
Methods: Autoantibodies targeting membrane repair proteins (dysferlin, annexins A5 and A6) in patient sera was measured using custom ELISAs. Membrane repair capacity was measured with a microscopic assay where mouse flexor digitorum brevis (FDB) muscles were bathed in 10.5 mM FM4-64, a lipophilic dye, during laser damage to the sarcolemma. The fluorescent intensity of the dye entering the injury site was used as a proxy for membrane repair. Lastly, AT (scurfy mouse lymph node cells into RAG1-/- mice) were injected with a commercial dysferlin antibody, 0.05 mg/kg weekly for 8 weeks, and muscle inflammation and serum creatine kinase (CK) concentrations were measured by histology or ELISA, respectively.
Results: We observed a significant increase in autoantibodies targeting dysferlin in IIM patient sera, with 77.78% one standard deviation and 26.67% of samples two standard deviations greater than the mean of healthy subjects. No such an increase in dysferlin autoantibodies was observed in systemic lupus systemic lupus erythematosus (SLE) patient serum samples. There was a positive correlation between dysferlin autoantibody levels and serum CK concentrations. This is likely caused by dysferlin antibodies compromising membrane repair as application of commercial Dysferlin antibodies (1:100 dilution) to wild type C57Bl/6 FDB muscles compromises sarcolemma repair when compared to a rabbit serum control. To test if these effects could enhance IIM pathology, we injected AT (scurfy mice lymph node cells into RAG1-/-) mice with control IgG or anti-Dysferlin commercial antibodies and observed a significant increase in the extent of inflammation and the concentration of CK in the serum of the antibody injected mice.
Conclusion: Our results reveal dysferlin autoantibodies may serve as a novel biomarker specific to IIM that can contribute to disease pathogenesis in this disease by decreasing sarcolemmal membrane repair capacity.
REFERENCES: [1] McElhanon KE, Young N, Hampton J, Paleo BJ, Kwiatkowski TA, Beck EX, Capati A, Jablonski K, Gurney T, Perez MAL, Aggarwal R, Oddis CV, Jarjour WN, Weisleder N. Autoantibodies targeting TRIM72 compromise membrane repair and contribute to inflammatory myopathy. J Clin Invest. 2020 Aug 3;130(8):4440-4455. DOI: 10.1172/JCI131721.PMID: 32687067
Acknowledgements: This research was supported by the National Institute of Health R01AR084518 and R56AR078800.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (