

Background: Sjögren’s disease (SjD) is a clinically and biologically heterogeneous disease. To date, no phase-III trial showed efficacy in reducing the symptoms or systemic activity of the disease and no immunomodulatory drug has been marketed. We have previously demonstrated using whole blood RNAseq data from the PRECISESADS project that SjD patients can be clustered into 4 distinct groups (endotypes) [1]. Cluster 1 was characterized by a unique interferon (IFN) signature, cluster 2 comprised healthy-like patients, cluster 3 was marked by IFN and B-cell signatures, cluster 4 was characterized by IFN, B-cell and neutrophil signatures.
Objectives: In this study, we hypothesized that patients in different clusters may have different responses to different drugs due to different therapeutic targets in each cluster.
Methods: Clinical, biological and transcriptomic data were obtained from three randomized controlled trials assessing hydroxychloroquine and leflunomide [2] (HCQ-LEF, including in our study 13 patients on HCQ-LEF and 5 on placebo), rituximab [3] (RTX, of whom 31 patients on RTX and 25 with placebo were included) or abatacept [4] (ABA, including 58 patients on ABA and 59 on placebo) versus placebo. Whole blood RNAseq was performed on samples collected at inclusion in the clinical trials. Patients were clustered into 4 endotypes using a semi-supervised Uniform Manifold Approximation and Projection for Dimension Reduction (UMAP) algorithm combined with a support vector machine (SVM) trained in respect to our previous work (Figure 1). We compared demographic features and disease characteristics between the 4 clusters. We then assessed response to therapy, defined by the STAR responder index [5], in the active treatment and placebo arms within each cluster. We analyzed this response rate in the 3 pooled trials, and then in each clinical trial individually.
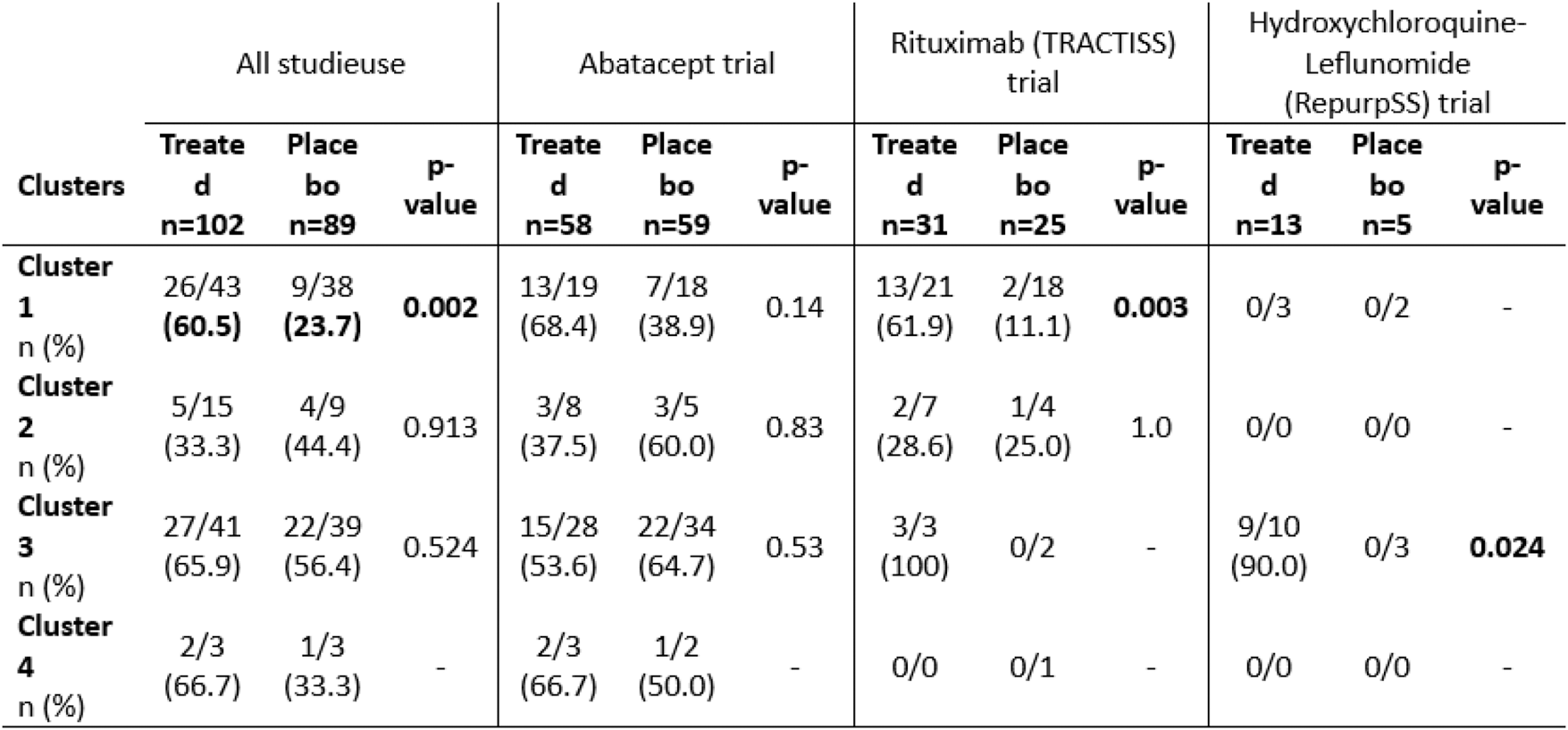
Results: Eighty-one subjects were clustered into cluster 1 (5, 39 and 37 from the HCQ-LEF, RTX and ABA trials, respectively), 24 were in cluster 2 (11 and 13 from the RTX and ABA trials), 80 were in cluster 3 (13, 5 and 62 from the HCQ-LEF, RTX and ABA trials) and 6 patients were in cluster 4 (1 and 5 from the RTX and ABA trials). ESSPRI scores did not differ between the 4 clusters (5.71 to 7.22; p=0.48). Unlike, ESSDAI was lowest in clusters 1 and 2 (6.36 and 6.62, respectively) and significantly higher in clusters 3 and 4 (8.81 and 10.67; p=0.003). When all 102 treated patients were pooled and compared to the 89 placebo patients, treated patients in cluster 1 were more likely to achieve a STAR response score than control patients (60.5% vs. 23.7%, p=0.002) (Table 1). A similar trend was seen in the RTX trial. In the HCQ-LEF trial, patients within cluster 3 who received the treatment were more likely to reach the STAR score. Whether the studies were pooled or analyzed separately, no treatment compared to placebo showed significant efficacy within cluster 2 (transcriptionally healthy-like patients).
Conclusion: The analysis of controlled trials with transcriptomic-based clusters of SjD patients highlights the lack of response to HCQ-LEF, RTX and ABA in the healthy-like patients. We suggest that these patients are less likely to respond according to STAR score, they may not be included into further controlled trials.
Stratification of the patients into 4 distinct clusters based on whole blood RNAseq. Abbreviations: LDA: Linear Discriminant Analysis. Footnotes: Linear Discriminant analysis of transcriptomic profiles of the patients from 3 different cohorts based on the clustering obtained from a support vector machine classifier
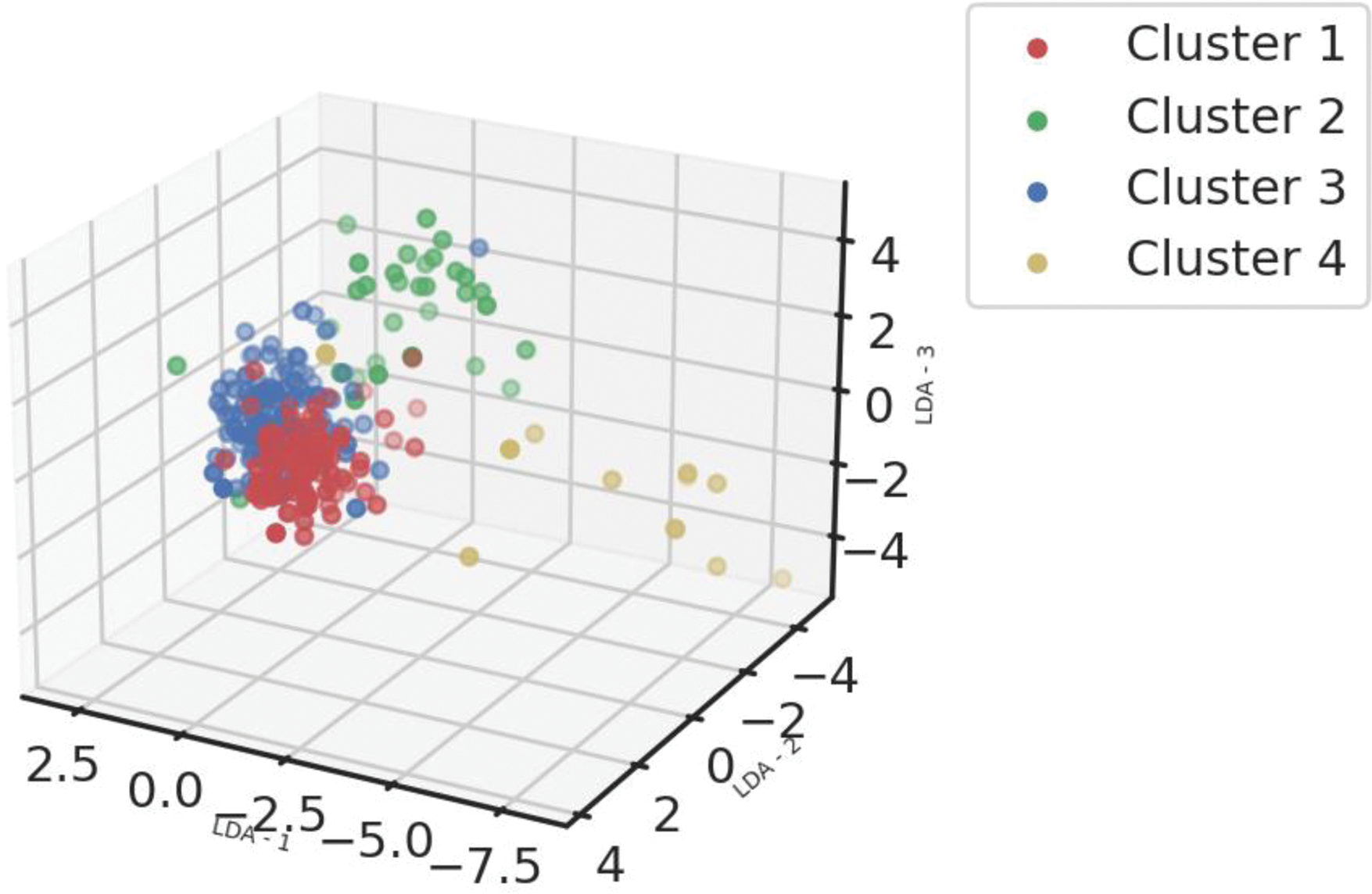
Table 1. STAR score response rate in patients with Sjögren’s disease clustered by endotypes in 3 different randomized controlled trials
REFERENCES:
[1] Soret, P. et al. A new molecular classification to drive precision treatment strategies in primary Sjögren’s syndrome. Nat Commun 12 , 3523 (2021).
[2] van der Heijden, E. H. M. et al. Leflunomide–hydroxychloroquine combination therapy in patients with primary Sjögren’s syndrome (RepurpSS-I): a placebo-controlled, double-blinded, randomised clinical trial. The Lancet Rheumatology 2 , e260–e269 (2020).
[3] Bowman, S. J. et al. Randomized Controlled Trial of Rituximab and Cost-Effectiveness Analysis in Treating Fatigue and Oral Dryness in Primary Sjögren’s Syndrome. Arthritis Rheumatol 69 , 1440–1450 (2017).
[4] Baer, A. N. et al. Efficacy and safety of abatacept in active primary Sjögren’s syndrome: results of a phase III, randomised, placebo-controlled trial. Ann Rheum Dis 80 , 339–348 (2021).
[5] Seror, R. et al. Development and preliminary validation of the Sjögren’s Tool for Assessing Response (STAR): a consensual composite score for assessing treatment effect in primary Sjögren’s syndrome. Ann Rheum Dis 81 , 979–989 (2022).
Acknowledgements: NIL.
Disclosure of Interests: Baptiste Chevet: None declared, Valerie Devauchelle-Pensec: None declared, Elena Pontarini: None declared, Valentin Baloche: None declared, Michele Bombardieri: None declared, Simon Bowman: None declared, Michael Barnes: None declared, Antoine Sreih: None declared, Jinqi Liu: None declared, Sheila Kelly: None declared, Antonia Christodoulou: None declared, Philippe MOINGEON: None declared, Laurence Laigle: None declared, Perrine Soret: None declared, Christelle Le Dantec: None declared, Jacques-Olivier Pers: None declared, Marta Alarcon-Riquelme: None declared, Guillermo Barturen: None declared, Xavier Mariette: None declared, Joel van Roon: None declared, Raphaèle Seror RS declares consulting fees from GSK, Boehringer, Novartis, Janssen, Abbvie., Implication in trials with GSK, Servier, USB, Novartis, Medimmune, Gaetane Nocturne: None declared, Divi Cornec: None declared, Nathan Foulquier: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (