

Background: Though it has been well recognized that virus play an important role in the pathogenesis of several autoimmune diseases such as Sjӧgren’s disease (SjD), less is known about the virome in those diseases especially in SjD. As salivary gland is the main exocrine gland involved in SjD, investigation of virome in saliva may more precisely reflect the association of virome change and disease state compared with intestinal virome.
Objectives: To identify viruses that highly presented and infected in patients with SjD, explore potential mechanisms in how those viruses may participate in SjD pathogenesis, providing new insights in how immune homeostasis is disrupted by viruses.
Methods: We performed a whole virome including DNA and RNA virus analysis in saliva based on the shotgun sequencing of 35 patients with SjD and 25 healthy control subjects. DESeq2 analysis was conducted to determine the altered virus. Spearman’s correlation between different virus species and SjD-related clinical indices was analyzed to explore the relationship between changes in the saliva virome and SjD. To confirm the infection of differentially presented viruses in saliva of patients with SjD, 150 oral swabs consisting mainly of epithelial cells in the oral cavity from patients with SjD and controls were collected, and nest PCR was performed. To test whether the virus species highly detected in SjD could provide cross-reactive antigens capable of initiating autoimmune responses, we extracted the sequences of autoantigen peptides proved to trigger autoimmune responses from the IEDB and then aligned them with the protein sequences of the viruses. The molecular mimicry peptides (mimotopes) were further synthesized for serum autoantibody detection. To determine whether the mimotopes could induce autoantibodies and Sjogren-related symptoms in vivo, C57BL/6 mice were immunized with the synthetic mimotopes linking bovine serum albumin (BSA).
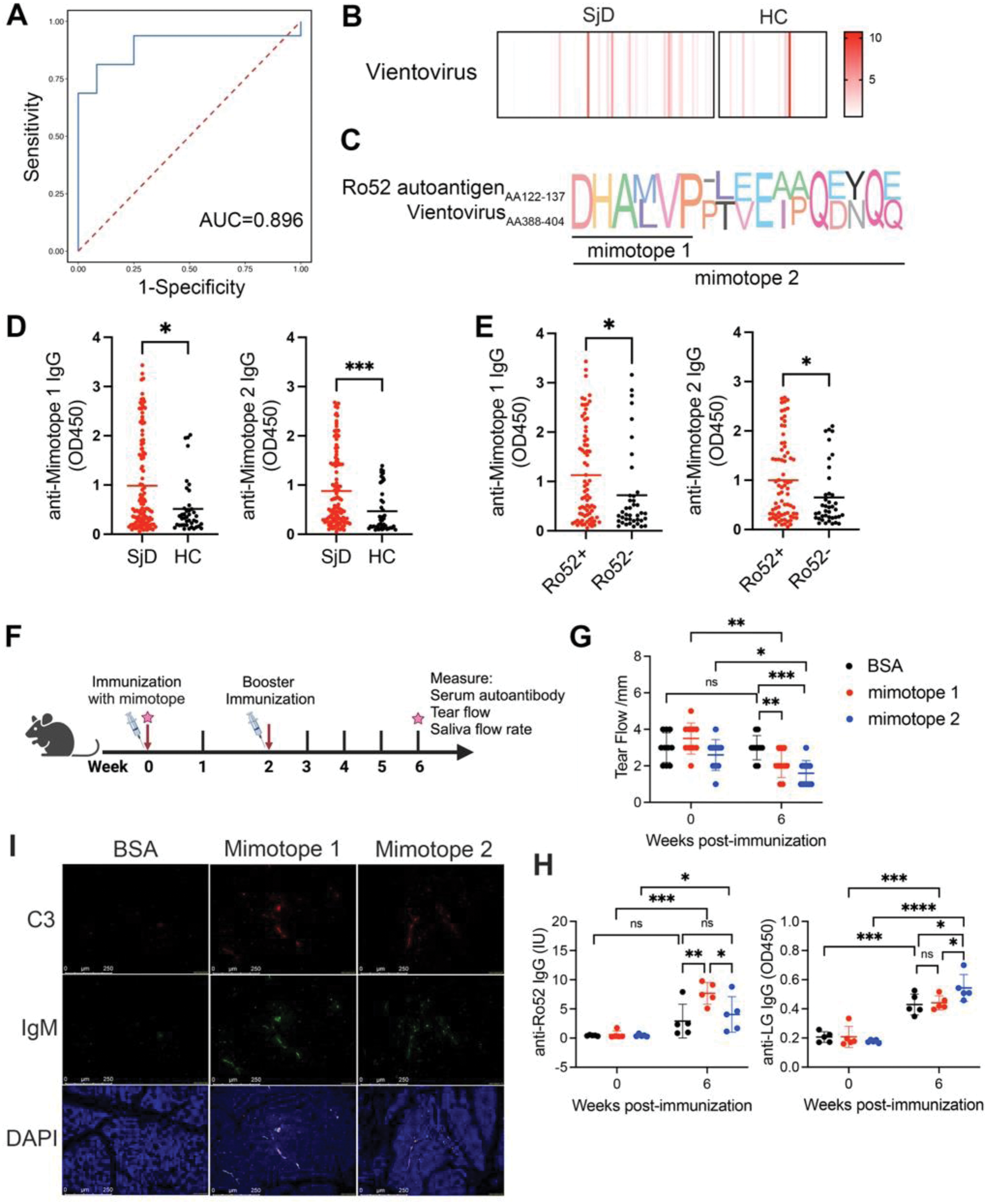
Results: A total of 586 known viral species were identified across all the samples, annotated as 43 eukaryotic DNA viruses, 43 eukaryotic RNA viruses and 500 prokaryotic viruses. Among them, 40 virus species were differently presented in the saliva of patients with SjD compared to HCs, 21 increased and 19 decreased in SjD. Based on the abundance of differentially presented viral species, a diagnostic model for SjD was established. The area under the curve (AUC) was 0.896, with a sensitivity and specificity of 91.67% and 68.75%, respectively (Figure 1A). Vientovirus, a species of the family Redondoviridae, was enriched in saliva of patients with SjD and validated in oral swabs (Figure 1B). The abundance of Vientovirus was significantly positively related to anti-Ro52 and negatively correlated with Schirmer’s test. The peptides from capsid protein of Vientovirus could mimic the B-cell autoepitopes of SSA/Ro52 (Figure 1C). Significantly elevated levels of autoantibodies against DHALVP (mimotope 1) and DHALVPPTVEIPQDNQQ (mimotope 2) were both revealed in the serum of patients with SjD (Figure 1D). There was significantly higher sero-reactivity to mimotope 1 and mimotope 2 in anti-Ro52-positive than in anti-Ro52-negative SjD patients (Figure 1E). Mice immunized with mimotope 1 and 2 showed significantly impaired tear secretion compared to those immunized with BSA (Figure 1F-G). At 6 weeks after initial immunization, the mimotopes significantly induced IgG against Ro-52, as well as antibodies against lacrimal gland (LG) protein (Figure 1H). Immunofluorescence staining showed increased deposition of IgM and C3 immune complexes in LG tissues of mice immunized with mimotopes compared to those in the BSA group, suggesting potential increased lacrimal immune pathology by Vientovirus mimotopes (Figure 1I).
Conclusion: We firstly depicted the disease-specific change of salivary virome associated with SjD, and unraveled the specific pathogenic role of newly annotated human-associated eukaryotic viruses Vientovirus in SjD, providing novel insights in how immune tolerance was breakdown in SjD.
(A) Based on the abundance of differentially presented viral species, a diagnostic model for SjD was established. The most discriminative viral species for SjD patients and HC were obtained by the XGBoost classifier. ROC curve analysis for the model with AUC value was shown. (B) Heatmap showing abundance of Vientovirus species in all oral swab samples. (C) Amino acid sequences alignment between the molecular mimicry peptides (mimotopes) from Vientovirus with the Ro52 autoantigen. (D) The levels of IgG against mimotope 1 and mimotope 2 were detected by ELISA in the sera of 115 SjD patients and 41 HCs. (E) The levels of IgG against mimotope 1 and mimotope 2 were detected by ELISA in the sera of SjD patients with anti-SSA/Ro52-positive (n=75) and anti-SSA/Ro52-negative (n=40). (F) Overview of the animal experiment. (G) Tear volume was measured by phenol red thread test. (H) Levels of IgG against Ro-52 or lacrimal gland (LG) protein were measured by ELISA. (I) Immunofluorescence staining of C3 and IgM in LG tissues of mice immunized with mimotopes.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (