

Background: There is a need for immunotherapy that sustains symptom remission without ongoing need for disease modifying drugs (DMARDs). In a phase 1b trial of antigen-specific tolerising immunotherapy in ACPA+ RA patients, expansion of peripheral blood CD4+ T cells recognising either collagen II 259-273 or vimentinCit64 59-71 was associated with reduced DAS28, suggesting that expanding autoreactive T cells may regulate RA under tolerogenic conditions [1]. In the BIOlogical Factors that Limit sustAined Remission in rhEumatoid arthritis (BIO-FLARE) study [2], 50% of patients who stop conventional synthetic (cs)DMARDs maintain “drug-free remission” (DFR) for at least 6 months, potentially also reflecting a state of immune tolerance.
Objectives: The current study compared the frequency and phenotype of peripheral blood CD4+ T cells recognising citrullinated (cit) vimentin in ACPA+ HLA-DRB1*04:01, *01:01 or *04:04+ RA patients who flared or remained in DFR 6 months after stopping csDMARDs with the aim to characterise the antigen-specific T cells in DFR.
Methods: Individuals with RA receiving methotrexate, sulfasalazine and hydroxychloroquine, singly or in combination (csDMARDs), and in clinician-defined remission (DAS28-CRP <2.4) were recruited into the BIO-FLARE study. Treatment was stopped and participants monitored over 24 weeks. Flare was defined as DAS28-CRP ≥3.2 at any visit or DAS28-CRP ≥2.4 twice within 14 days. PBMC were analysed using a 32 marker spectral flow panel, incorporating HLA-DRB1*04:01/01:01-VimentinCit64 59-71 or HLA-DRB1*04:04-VimentinCit71 66-78 tetramers. Data were analysed using Wilcoxon signed-rank tests.
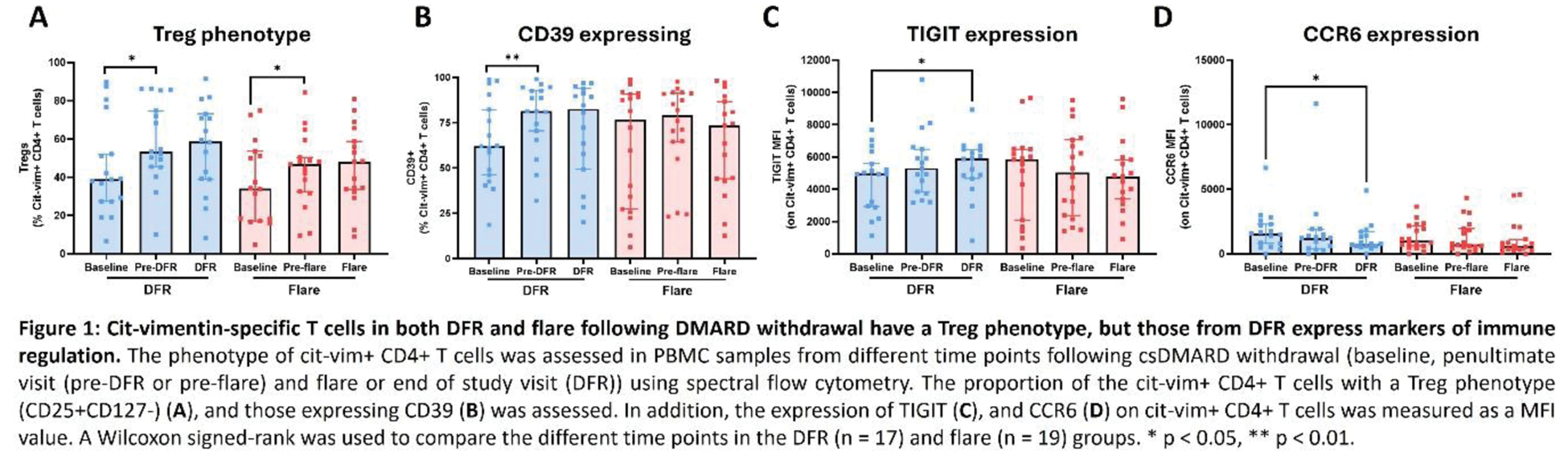
Results: Nineteen flaring patients were compared with 17 patients remaining in DFR 24 weeks after stopping therapy. At baseline the number and phenotype of cit-vimentin-specific CD4+ T cells was comparable in both groups, except that CD38 expression was higher in the group subsequently maintaining DFR. In each group, cit-vimentin-specific CD25 + CD127 - Treg increased and expression of CD55 decreased over time (Figure 1A). At flare or the preceding time point a lower proportion of cit-vimentin-specific CD4+ T cells expressed CD161, fewer were naïve, and CXCR3 expression was reduced. In the DFR group, cit-vimentin-specific CD4+ T cells expanded, the proportion expressing CD39 increased, whilst expression of TIGIT increased and CCR6 decreased (Figure 1B-D).
Conclusion: Together these data suggest that, in patients achieving DFR, there is ongoing cit-vimentin autoantigen presentation and T cell activation under tolerogenic conditions, expanding antigen-specific CD4+ T cells expressing markers of immune regulation and anergy. Reduced circulating CD161+CXCR3hi antigen-specific T cells during flare suggests migration of effector memory autoreactive T cells to synovial inflammatory sites.
REFERENCES: [1] Sonigra A et al. JCI Insight 2022.
[2] Rayner F et al. BMC Rheumatol. 2021.
Acknowledgements: NIL.
Disclosure of Interests: Amy E. Anderson: None declared, Henrique Lemos: None declared, Hendrik Nel: None declared, Sofia Sorbet Santiago: None declared, Jia Yi Hee: None declared, Fiona Rayner: None declared, Abbie Degnan: None declared, Imogen Wilson: None declared, Julie Diboll: None declared, Jasmine Sim: None declared, Andrew Melville: None declared, Stefan Siebert AbbVie, Amgen, AstraZeneca, Janssen, Novartis, Pfizer, Syncona, Teijin Pharma and UCB, BMS, Boehringer-Ingelheim, Eli Lilly, GSK, Janssen and UCB, Iain B. McInnes: None declared, Carl S Goodyear: None declared, Catharien Hilkens: None declared, Andrew Filer: None declared, Karim Raza: None declared, Christopher D Buckley: None declared, Hugh Reid: None declared, Kenneth F Baker Modern Biosciences, Pfizer and Genentech, Arthur Pratt: None declared, Jamie Rossjohn: None declared, Ranjeny Thomas: None declared, John D Isaacs: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (