

Background: The emergence of subclinical synovitis on imaging in anti-cyclic citrullinated peptide positive (anti-CCP+) individuals with musculoskeletal (MSK) symptoms represent a late stage of the pre-rheumatoid arthritis (RA) disease continuum and is a strong predictor of imminent progression to clinical arthritis. Importantly, it represents the transition from systemic autoimmunity to synovial inflammation, the so called ‘second hit’ of RA. The circulating immune cell perturbations that drive the onset of synovial inflammation remain unclear. Their elucidation is likely to provide insights into potential targets for preventive intervention.
Objectives: To characterize circulating immune cell subsets and their gene expression profiles in high-risk anti-CCP+ individuals, with and without subclinical synovitis, using single-cell RNA sequencing (scRNAseq).
Methods: High-risk anti-CCP+ at risk individuals (n=40) who had arthralgia, early morning stiffness >30 mins and CCP >300U/ml were selected from the prospective Leeds CCP cohort. Of these, 22/40 progressed to RA within six months of baseline visit and 18 did not progress at 2-year follow up. Progressors were separated into those with subclinical synovitis on ultrasound (US+) at baseline visit (n=11) and those without subclinical synovitis on ultrasound (US-) (n=11). Single-cell RNA sequencing (scRNA-seq) was performed on cryopreserved peripheral blood mononuclear cells (PBMCs) collected at baseline to assess immune cell composition and gene expression profiles. Cell clusters were derived using Seurat v5, with highly variable genes selected for principal component analysis (PCA) and followed by clustering with the Leiden algorithm. Subsequently, UMAP were generated for further dimensionality reduction and visualization of cell clusters. These cell clusters were first mapped to the COMBAT (COVID-19 Multi-Omic Blood ATlas) reference dataset for the identification and classification of immune cell subsets and subsequently refined using Seurat’s FindAllMarkers function to identify specific marker genes for each cluster and comprehensively annotate immune cell populations. Differential gene expression analysis between cell clusters and conditions was conducted using DESeq2 on pseudobulk aggregates, enabling the identification of condition-specific transcriptomic signatures.
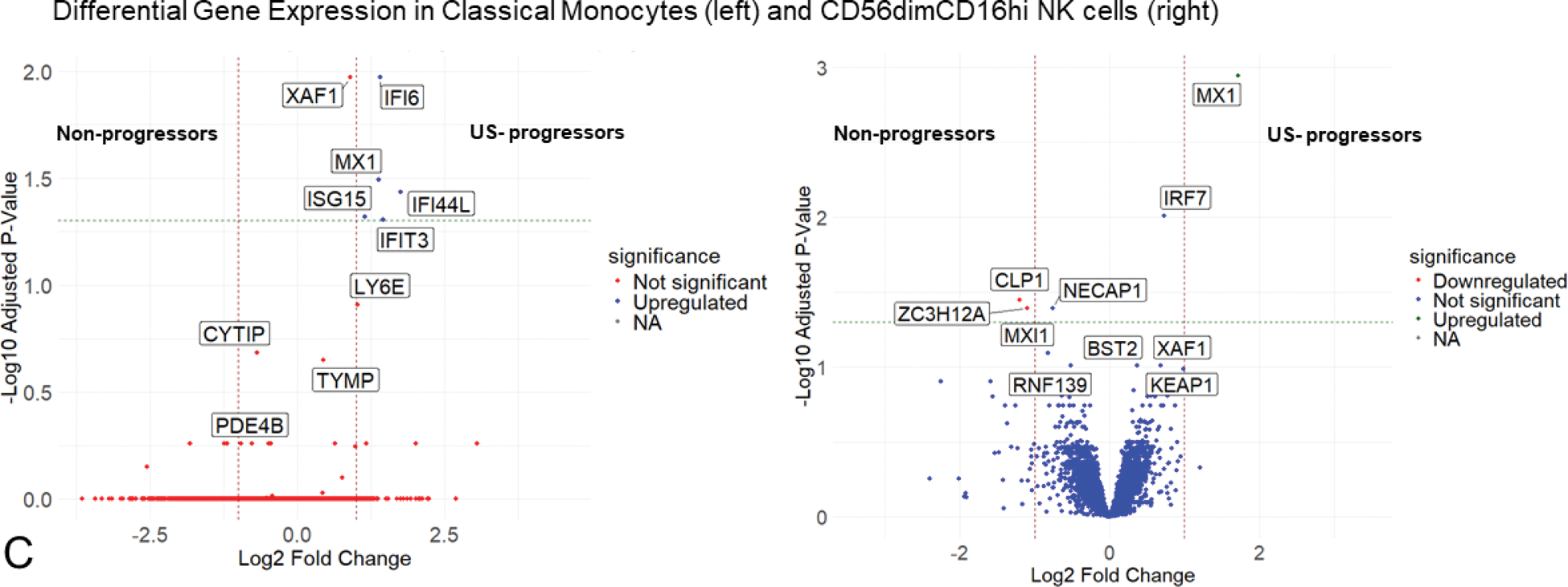
Results: Baseline characteristics are summarized in Table 1. Using unsupervised Leiden clustering at resolution 1, 31 distinct cell subsets of innate and adaptive immune cells were identified (Figure 1A). Among these, four subsets displayed significant proportional differences across the three patient groups (US+ progressors, US- progressors, non-progressors). Circulating classical monocytes (CD14+CD16-) were significantly elevated in US- progressors compared to US+ progressors (p=0.034). The US+ progressors exhibited significantly higher proportions of CD8+ TEMRA (GZMB+PRF1+CCR7-) cells (p=0.039), including a distinct CMC1+ TEMRA (0.047) population. Non-progressors showed significantly higher proportions of memory B cells compared to US- progressors (0.049) (Figure 1B). Differential gene expression analysis among the 31 immune cell subsets revealed that US- progressors had significant upregulation of type 1 interferon (IFN) stimulated genes— MX1 , ISG15 , IFI6 , IFI44L , and IFIT3 —in classical monocytes compared with non-progressors (Figure 1C). Similarly, the CD56dimCD16hi NK cell subset in US- progressors showed significant upregulation of MX1 compared with non-progressors. Comparisons between US- progressors, and both US+ progressors and non-progressors revealed no significant differences in cellular proportions as well as gene expression in all other cell subsets.
Conclusion: In CCP+ individuals who go on to develop RA within 2 years, altered circulating innate immune cell gene expression occurs prior to the onset of subclinical and clinical synovitis. Specifically, we identified an increased frequency of circulating classical monocytes with upregulated Type I interferon (IFN) stimulated genes. This may represent a peripheral blood signature associated with the transition to clinical arthritis in at risk individuals and suggests a potential early role for Type 1 IFN in arthritis development.
REFERENCES: NIL.
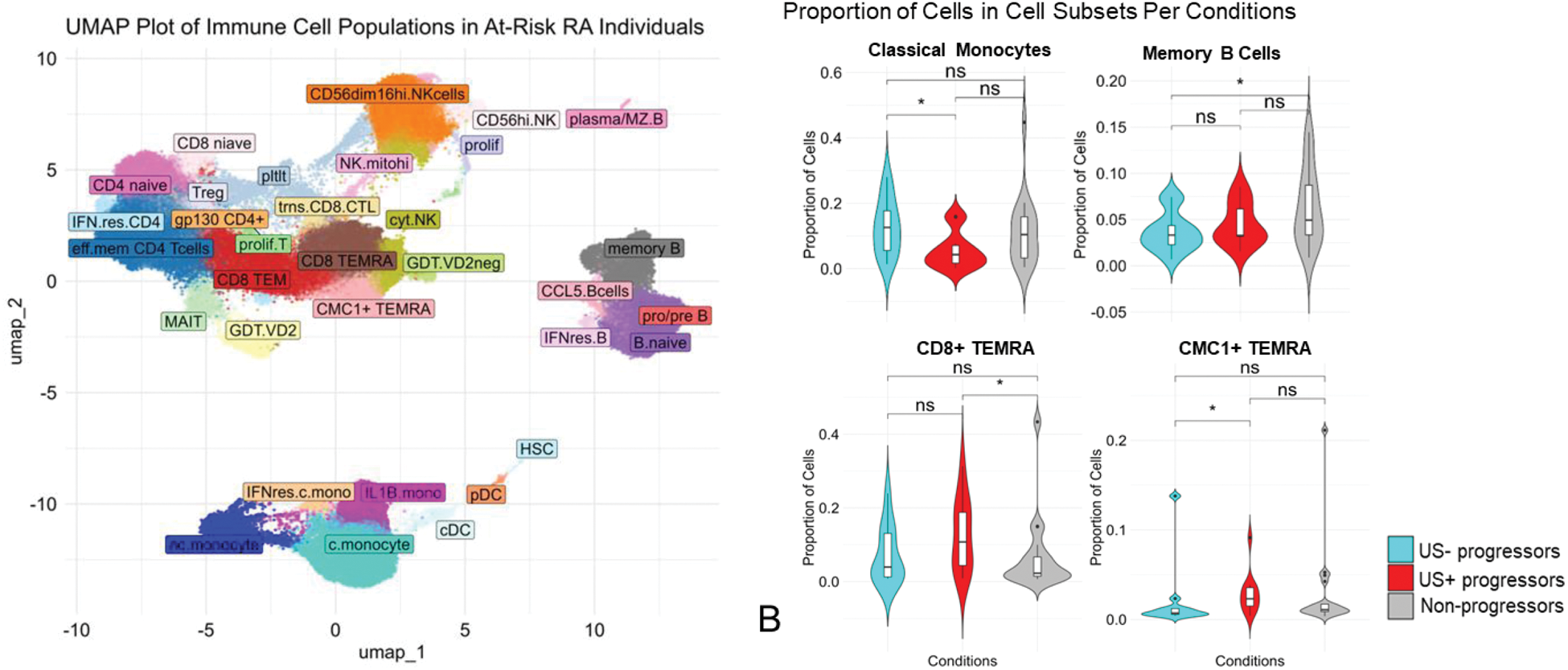
Immune Cell Populations and Differential Gene Expression in Anti-CCP+ Individuals at Risk of Imminent Progression to RA. (A) UMAP plot showing 31 immune cell subsets in PBMCs. (B) Violin plots reveal differences in immune cell proportions: classical monocytes were elevated in US- progressors, while CD8+ TEMRA and CMC1+ TEMRA were higher in US+ progressors. Non-progressors had more memory B cells. (C) Volcano plots show differential gene expression in classical monocytes and NK cells, with significant upregulation in US- progressors.
Baseline characteristics of the three anti-CCP+ at-risk groups.
| Variables | US-negative progressors (n=11) | US-positive progressors (n=11) | Non-progressors (n=18) | P-value | |
|---|---|---|---|---|---|
| Anti-CCP value (U/ml)
| 145 (SD: 123) | 195 (SD: 100.51) | 98 (SD: 94.98) | ns | |
| Time to progression (m)
| 3.43 (SD: 1.87) | 1.87 (SD: 0.94) | ≥2 years follow-up | - | |
| Age (years)
| 47.73 (SD: 17.93) | 46.45 (SD: 18.14) | 54.5 (SD: 11) | ns | |
| CRP levels (mg/L)
| 7.86 (SD: 3.6) | 12.2 (SD: 22.44) | 5.79 (SD: 6.2) | ns | |
| ESR (mm/hr) (mean) | 20.78 (SD: 9.99) | 23.11 (SD: 19.35) | 10.5 (SD: 9.91) | ns | |
| Sex | F% | 63.63 | 54.5 | 55.5 | - |
| M% | 36.36 | 45.4 | 44.4 | ||
| Smoking | Yes% | 63.63 | 54 | 66.66 | ns |
| No% | 27.27 | 36 | 33.3 | ||
| HLA-DRB1+ | Yes% | 54.54 | 63.63 | 100 | - |
| No% | 45.45 | 36.36 | 0) | ||
Acronyms - (Anti-CCP:Anti-cyclic citrullinated peptide, m:month, CRP: C-reactive proteins, ESR: erythrocyte sedimentation rate, F:Female, M:Male, US:Ultrasound)
Acknowledgements: NIL.
Disclosure of Interests: Fareeha Tariq: None declared, Weiyu Ye: None declared, Paul Martin: None declared, Xiang Sun: None declared, Sophie MacKay: None declared, Dylan Muldoon: None declared, Laurence Duquenne: None declared, Andrea Di Matteo: None declared, Kate Harnden: None declared, Katie Mbara: None declared, Madhvi Menon: None declared, Maya Buch: None declared, Benjamin Fairfax: None declared, Darren Newton: None declared, Paul Emery Abbvie, Activa, Astra-Zeneca, BMS, Boehringer Ingelheim, Galapagos, Gilead, Immunovant, Janssen, Lilly, Novartis, Abbvie, BMS, Lilly, Novartis, Pfizer, Samsung, Kulveer Mankia Abbvie, ALLin Bio, Astra Zeneca, UCB, Lilly, Galapagos, Serac Healthcare, Zura Bio, Deepcure, Gilead, Lilly, Serac Healthcare, Astra Zeneca, Deepcure.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (