

Background: Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by persistent joint inflammation and structural damage. Mucosal-associated invariant T (MAIT) cells are innate-like T lymphocytes able to produce diverse cytokines and cytotoxic mediators, such as granzyme B. Reduced frequencies and altered phenotypes of MAIT cells have been observed in various autoimmune diseases. Their ability to interact with microbiota and accumulate in inflamed tissues highlights their potential role in the pathogenesis of RA.
Objectives: To investigate the multifaceted involvement of MAIT cells in RA, focusing on their phenotypic and functional changes, their role in local and systemic inflammation, and their potential as therapeutic targets.
Methods: MAIT cell frequency and phenotype were analyzed by flow cytometry in fresh peripheral blood from 70 RA patients and 41 healthy donors (HD), with a focus on activation, exhaustion, apoptosis, proliferation markers, and cytokine secretion after 6 hours of PMA/ionomycin stimulation. Additionally, MAIT cells from synovial fluid were compared to those from peripheral blood in paired samples from 19 RA patients. These cytometry data were complemented by single-cell RNA sequencing (scRNAseq) data from 6 paired RA samples (blood and synovial fluid) and 4 blood HD samples. The interaction between MAIT cells and fibroblast-like synoviocytes (FLS) was explored through co-culture experiments. Finally, the impact of MAIT cell modulation was assessed in two mouse models of arthritis: mBSA-induced arthritis (an acute arthritis model) and chronic erosive arthritis in TNF-transgenic mice (Tg197). MAIT cells were modulated using MR1 KO mice (which lack MAIT cells) and through the intraperitoneal administration of Ac6FP, an inhibitor of MAIT cell activation.
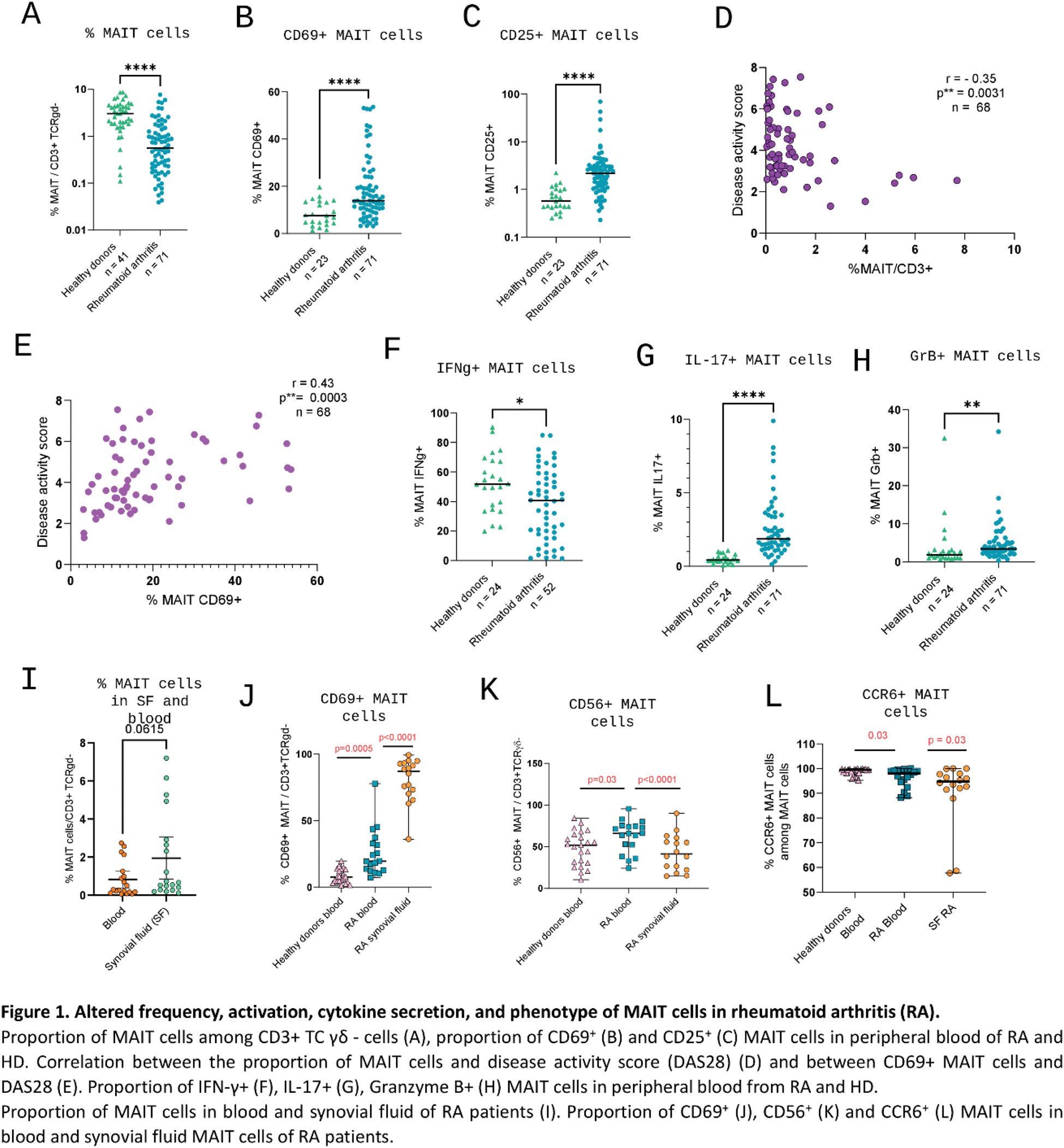
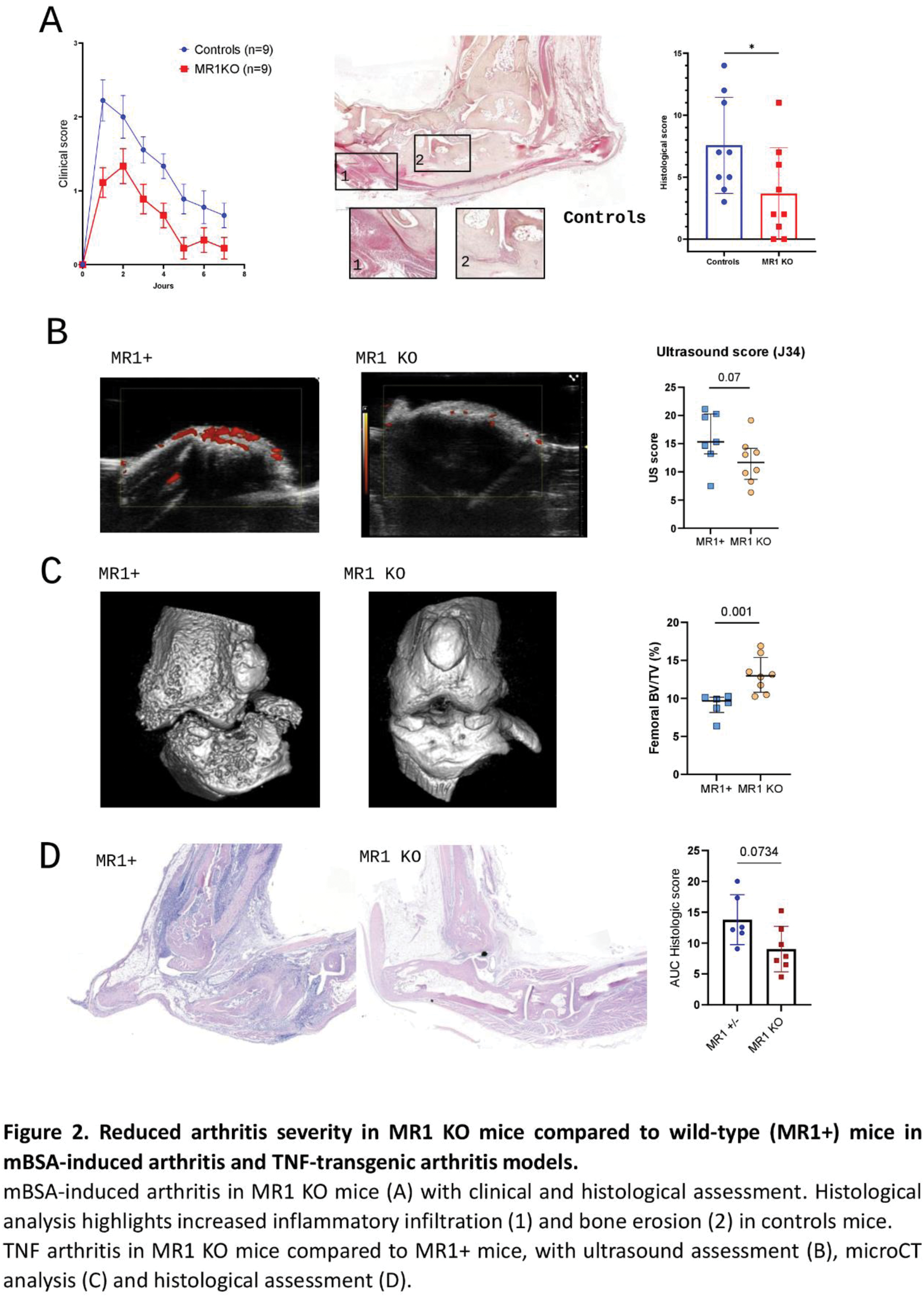
Results: MAIT cell proportion was significantly reduced in RA patients compared to HD (0.56% vs 3.1%, p<0.001, Figure 1A). Circulating MAIT cells displayed an activated phenotype in RA compared to HD, with increased proportion of CD69+ (13.9% vs 7.6%, p<0.001, Figure 1B) and CD25+ (2.2% in RA vs 0.57%, p<0.001, Figure 1C) MAIT cells. Markers of exhaustion (Tim-3 MAIT cell proportion, 0.56% vs 0.26%, p=0.017) and proliferation (Ki67, 1.3% vs 1%, p=0.026) were also increased in RA. DAS28 scores negatively correlated with MAIT cell proportions (r=-0.35, p=0.003, Figure 1D) and positively correlated with CD69+ MAIT cells (r=0.43, p<0.001, Figure 1E). RA MAIT cells secreted significantly more IL-17 (1.9% vs 0.4%, p<0.001, Figure 1G) and granzyme B (3.4% vs 1.9%, p=0.003, Figure 1H). Conversely, Th1 cytokine secretion was reduced, with lower proportions of IFN-γ+ MAIT cells (40.6% vs 52%, p=0.016, Figure 1F). ScRNA sequencing revealed increased cytotoxicity of circulating MAIT cells in RA, with upregulation of PRF1 and KLRC2 genes compared to HD. Finally, RA MAIT cells exhibited a migratory profile favoring infiltration into inflamed tissues, characterized by upregulation of genes coding for chemokines such as CCL3L1 and CCL4. MAIT cells in synovial fluid (SF) were more abundant compared to blood (0.67% in synovial fluid vs. 0.3% in blood; p =0.062, Figure 1I). This enrichment was particularly pronounced in early arthritis (<3 weeks), with a median SF/blood MAIT cell ratio of 5.4, compared to 0.7 in arthritis lasting >3 weeks ( p =0.001). Synovial MAIT cells were more activated, with higher CD69 expression (87% vs 20% in blood, p<0.001, Figure 1J) and a trend for increased CD25 expression (7% in SF vs 6% in blood, p=0.159). Interestingly, these cells displayed reduced expression of CD56 and CCR6 markers (41% in SF vs 66% in blood, p<0.001 and 94.8% in SF vs 98% in blood, p=0.034, respectively, Figure 1K-L), suggesting phenotypic changes after migration into the synovium. ScRNA sequencing further confirmed the activated profile of synovial MAIT cells, with upregulation of CD44, FAM102A, FAM102B, and KLF6 genes in SF MAIT cells compared to blood MAIT cells. Additionally, scRNA-seq data supported the hypothesis of circulating MAIT cell migration into synovial tissue, with a specific upregulation in SF MAIT cells of migration-associated genes such as ITGA1 and ITM2C, as well as tissue infiltration genes like CCL5. In co-culture, activated MAIT cells enhanced the activation of synovial fibroblasts, with increased expression of CD54 (97% vs 35% in FLS alone, p=0.005), CD106 (31% v 13% in alone FLS, p=0.047), and PDPN (87% vs 80% in alone FLS, p=0.034). In mouse models, MR1 KO mice subjected to mBSA-induced arthritis exhibited alleviated disease severity compared to wild-type mice, both clinically (median AUC clinical score: 4 vs. 9.5, p=0.007) and histologically (median score: 2 vs. 7, p=0.037, Figure 2A). In Tg197 mice, MR1 KO mice exhibited reduced inflammation on joint power doppler ultrasound (score: 11.7 vs. 15.3, p=0.07, Figure 2B) and less structural damages, evidenced by increased bone volume at microCT (BV/TV: 13% vs. 9.6%, p=0.001, Figure 2C) and a lower histological score (7.4 vs. 12.8, p=0.07, Figure 2D). MAIT cell inhibition using Ac6FP was associated in the mBSA arthritis model with reduced clinical severity (AUC clinical score: 7.7 vs. 14.5, p=0.07) without histological differences. Similarly, Ac6FP-treated Tg197 mice exhibited a trend toward lower clinical scores (AUC clinical score: 9.6 vs. 18, p=0.141).
Conclusion: Our findings reveal a pivotal role for MAIT cells in the pathogenesis of RA. These cells are activated and exhibit phenotypic alterations in the peripheral blood of RA patients. They accumulate in the synovial fluid, where they contribute to inflammation by interacting with synovial fibroblasts. Modulating MAIT cells markedly improved arthritis severity in preclinical models, highlighting their potential as therapeutic targets in RA.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (