

Background: The deterioration of gut-barrier integrity facilitates the onset of arthritis and suggests the restoration of gut-barrier homeostasis as a novel therapeutic strategy for RA. The self-renewal and differentiation of intestinal epithelial stem cells (ISCs) are crucial for maintaining the integrity and functionality of the intestinal barrier. Previous works suggestd that butyrate has a substantial preventative effect on RA by targeting osteoclasts as well as cellular and humoral immune responses. Additionally, butyrate serves as the primary energy source for colonic substance metabolism.The mechanism via which butyrate alleviates the advancement of RA by promoting the proliferation of ISCs thereby improving the intestinal barrier remains undisclosed.
Objectives: To elucidate the mechanism that butyrate mitigates the progression of RA by enhancing the self-renewal and differentiation of colonic stem cells and reinstating the intestinal barrier.
Methods: Constructed with male DBA/1 mice, the established collagen-induced arthritis (CIA) mice received butyrate (200 mg/kg/d, po) over a period of 4 weeks (n = 6) (Figure 1). The arthritis score was estimated bi-daily. The posterior ankles were harvested for hematoxylin and eosin (HE) staining and micro CT to evaluate the synovial inflammation and bone erosion. The spleen and the joint draining lymph nodes (DLNs) were collected for flow cytometric analysis to examine the alterations in immune cell subsets following butyrate therapy. Colon and small intestine tissues were gathered for immunofluorescence (IF) and HE staining. An investigation of the additional mechanism by which butyrate improves colon ISC function in RA was conducted using single-cell RNA sequencing (scRNA-seq).
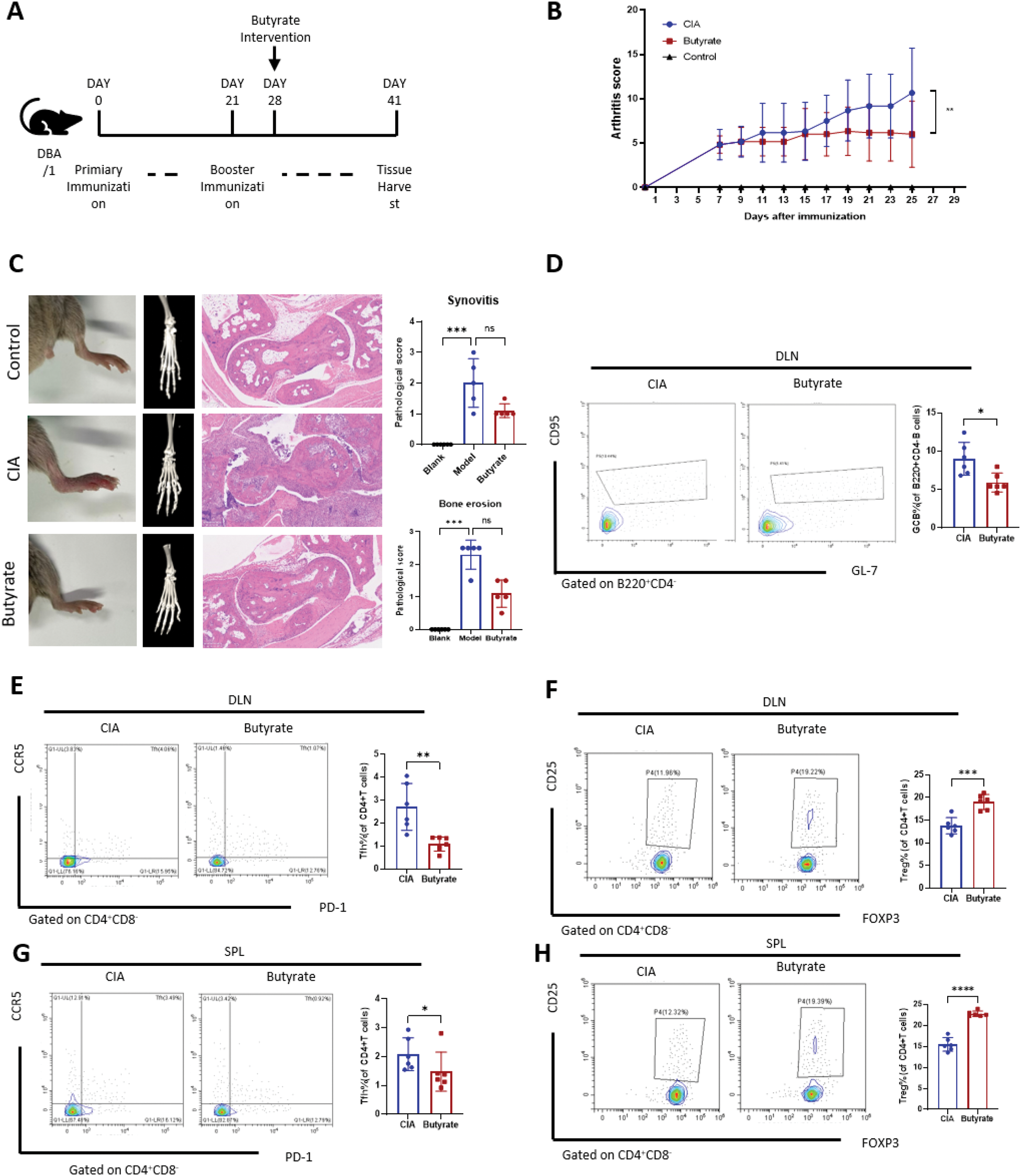
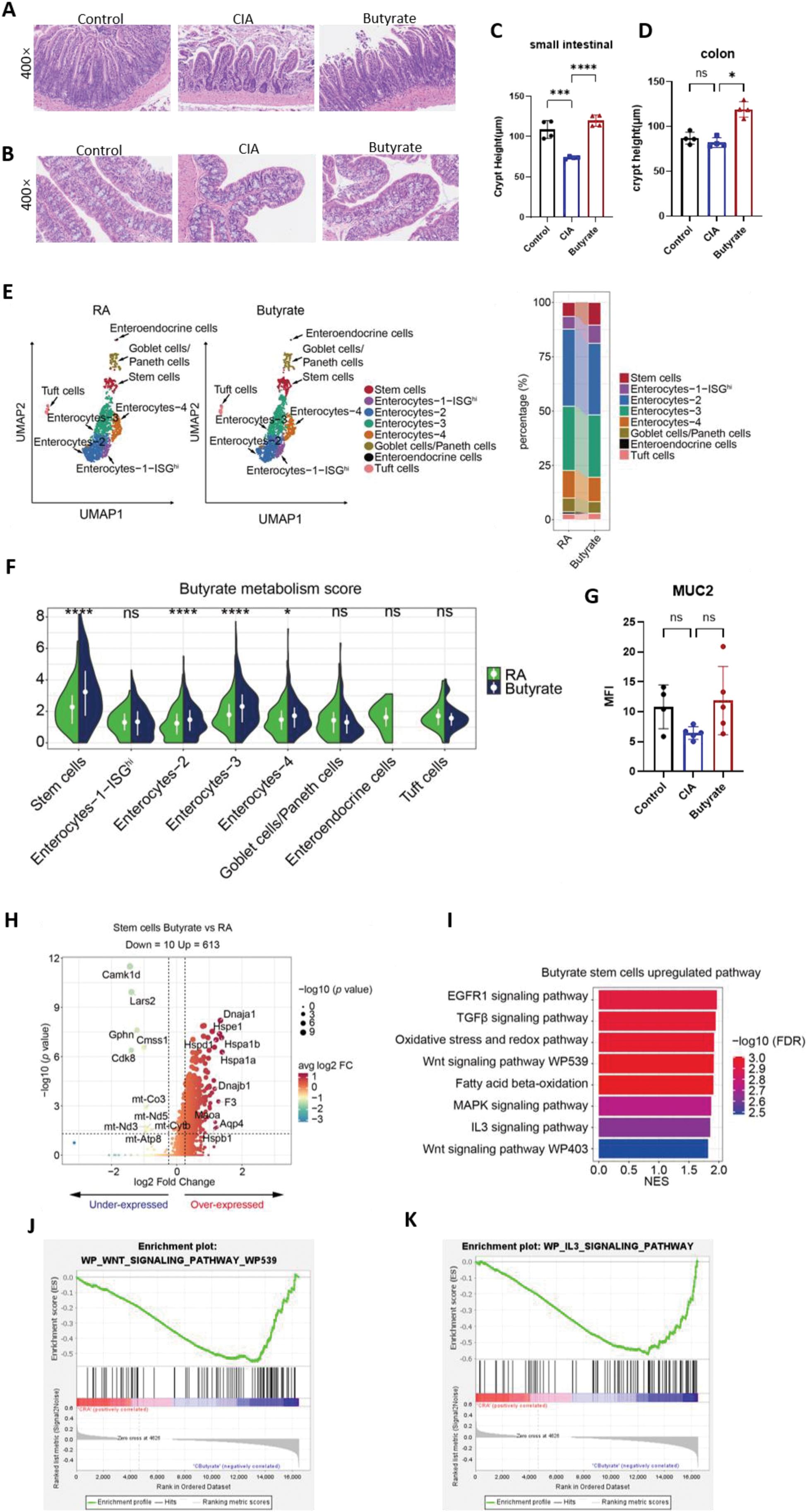
Results: Intragastric butyrate administration was initiated after two immunizations (Figure 4A). Compared to CIA model fed a normal chow diet, butyrate treatment reduced the arthritis score prominently (P = 0.001, Figure 4B). A downward trend in the severity of joint inflammation and bone erosion was observed (Figures 4C). Flow cytometry analysis of B cells revealed that butyrate administration reduced the proportion of CD4+CD220+CD95+ germinal center B (GCB) cells in the joint draining lymph nodes (DLNs) (Figure 4D). In T cells, butyrate administration reduced the proportion of CD4+CD8−CXCR5+PD-1+ T follicular helper (TFH) cells in both the DLNs (P < 0.001; Figure 5E) and the spleen (P < 0.05; Figure 4E). Furthermore, a significant increase in the proportion of CD4+CD25+Foxp3+ regulatory T cells (Tregs) was observed in both the DLNs (P < 0.001; Figure 5F) and the spleen (P < 0.001; Figure 4F). These results confirm the protective effects of intestinal butyrate in the development of rheumatoid arthritis (RA). In the CIA model, the crypt height of the intestine was decreased compared to the normal group, but butyrate supplements can restore the crypt height backup to normal levels (P < 0.0001, Figure. 2A and C). Although there is no difference in colon crypt height between CIA model and the control group, butyrate can enhance it (P < 0.05, Figure 2E). The scRNA-seq of colon tissue indicated that the proliferation of ISCs in the butyrate group was greater than CIA group (Figure 2B and D). GSVA analysis showed that the butyrate metabolism score of ISCs increased subsequent to the butyrate supplementation (P < 0.0001, Figure. 2F). As anticipated, following butyrate administration, ISCs have a distinct gene expression profile compared to those in the CIA paradigm (Figure 2H). The upregulated genes predominantly participate in the cellular stress response, particularly the heat shock protein family, which is crucial for cellular adaptation to oxidative stress, inflammation, and tissue repair. The enriched upgrading pathways were assessed using KEGG and GSEA, identifying Wnt signaling pathways and IL3 signaling pathways (Figure 2J to L) as the potential mechanisms by which butyrate facilitates the differentiation of ISCs.
Conclusion: Butyrate can improving the intestinal barrier via promoting the proliferation of intestinal stem cells and thereby alleviates the advancement of rheumatoid arthritis.
REFERENCES: NIL.
Butyrate suppresses immune responses and reduces joint inflammation and bone destruction in mice. (A) Scheme of the experimental design for a butyrate diet using the classic CIA mouse model of RA. (B) Arthritis scores in CIA mice with or without butyrate supplementation.(C) Arthritis and bone erosion of the posterior ankles by photos, micro-CT (middle) and hematoxylin and eosin (H&E)–stained (left) analysis (Zoom in 50 times).(D to H) Representative flow cytometry plots with graphs showing the frequency of B220+ CD4− CD95+ GL-7+ germinal center B cells (D), CD4+ CD25+ Foxp3+ Tregs (F and H), and CD4+ CXCR5+ PD1+ Bcl6+ TFH cells (E and G). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Butyrate suppresses immune responses and reduces joint inflammation and bone destruction in mice. (A and B) Tissues from the small intestine and colon subjected to HE staining. (C and D) Graphs of crypt height of small intestine and colon. (E) Umap plot of intestinal epithelial cells (IECs) (left) beside a stacked histogram displaying the quantities of each type of IECs (right) classified by CIA and butyrate group. (F) GSVA analysis of butanoate metabolism pathway in intestinal stem cells. (G) The graph displays the mean fluorescence intensity (MFI) of MUC2 immunofluorescence. (H) Volcano plot depicting transcript fold change of ISCs before and after butyrate administration versus P value. (I) Bar plots showing the DEG (differential expressed genes) enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways of ISCs exposed to butyrate administration. (J and K) GSEA plot. (C, D and G). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Acknowledgements: Thanks to all the participating researchers and the experimental animals.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (