

Background: The shared epitope (SE), a genetic sequence coding for a 5-amino acid motif within the peptide-binding groove of HLA-DRβ1, represents the strongest genetic factor associated with both susceptibility and prognosis of rheumatoid arthritis (RA). However, the mechanisms of action underlying its association with clinical outcome measures remain poorly understood, limiting the development of tolerogenic therapies for precision medicine targeting the right autoantigens, the right cell types, at the right time. HLA-DRB1*04:01 is the most common SE allele and its unique structure facilitates the presentation of citrullinated self-peptides to CD4 + T cells.
Objectives: To explore cellular mechanisms of autoimmunity underlying the association between HLA-DRB1*04:01 and RA outcome.
Methods: The Biologics in Rheumatoid Arthritis Genetics and Genomics Study Syndicate (BRAGGSS) recruits RA patients who have failed to respond to conventional synthetic Disease-Modifying Anti-Rheumatic Drugs (DMARDs) from 53 centres across the UK. Clinical data is collected before initiation of biologic drugs and at 3, 6 and 12 months post-biologic treatment. Patients prescribed a second line biologic drug (or higher order) can be re-recruited into BRAGGSS providing an additional 12 months of follow up. Blood samples for the extraction of DNA and peripheral blood mononuclear cells are taken at recruitment. In parallel, we also recruited control individuals without any history of rheumatologic conditions into the National Repository of Healthy Volunteers (NRHV). Genotyping was performed using the Illumina Human Core Exome array, followed by imputation of 4-digit HLA alleles with SNP2HLA. Six different fluorophore-labelled HLA-DRβ1*04:01 tetramers loaded with 6 different citrullinated autoantigenic peptides originating from Vimentin, Cartilage Intermediate Layer Protein and α-Enolase were incorporated into a 15-colour flow cytometry panel, in order to quantify and deeply immunophenotype citrulline-specific CD4 + T cells. Anti-citrullinated protein/peptide antibody (ACPA) status and titres were derived from clinical notes and also determined in-house by ELISA. A control tetramer loaded with an influenza peptide was used to quantify influenza-specific CD4 + T cells.
Results: Between January 2015 and December 2021, we recruited 310 subjects (212 RA patients and 98 NRHVs). The SE was strongly associated with RA susceptibility (OR=2.2, p=2.9E-05), as was HLA-DRB1*04:01 (OR=2.8, p=1.2E-04). The frequency and immunophenotype of citrulline-specific CD4 + T cells was determined in 63 HLA-DRB1*04:01 + participants (46 patients, 17 NRHVs). Citrulline-specific CD4 + T cells were significantly increased in RA patients (p=3.xE-04), even in those without humoral immunity against citrulline (ACPA-negative), were associated with disease duration (p=0.02), CRP (p=0.03) and disability (HAQ score p=0.008). The frequency of citrulline-specific CD4 + T cells was significantly reduced after methotrexate treatment (p=0.01; Figure 1), an effect not observed on influenza-specific T cells and not observed for other DMARDs. The frequency of citrulline-specific CD4 + T cells was comparable in patients who went on to respond well to their first biologic treatment and in healthy (Figure 2). Within patients who started their first line biologic drug, the frequency of citrulline-specific CD4 + T cells was significantly associated with future EULAR response (p=0.027; Figure 2). Citrulline-specific CD4 + T cells were increased by 10 times, up to the level of influenza-specific CD4 + T cells, in patients with difficult-to-treat (D2T) RA, or in general in patients starting a second line (or higher order) biologic treatment, regardless of their outcome (p=0.027). Therefore, citrulline-specific CD4 + T cells increased in frequency with disease course when patients failed to response to biologics (trend p-value = 2.2E-04). Surprisingly, citrulline-specific CD4 + T cells were not autoimmune-associated Tph cells, but displayed a phenotype nearly identical to influenza-specific CD4 + T cells, with a predominance of PD1 neg CXCR5 neg , HLA-DR + CD27 + and T EM cells.
Conclusion: Cellular autoimmunity against citrulline is present in all HLA-DRB1*04:01 + patients, even in the in the absence of humoral autoimmunity and associates with multiple outcome measures. It is suppressed by methotrexate, associates with response to biologic drugs, and increases in frequency along the patient journey towards treatment resistance. The immunophenotype of anti-citrulline CD4 + T cells is similar to an anti-viral phenotype. These observations are compatible with a cardinal role for cellular autoimmunity against citrulline in the aetiology, maintenance, progression and resistance to treatment in RA, increasing confidence that citrulline-specific CD4 + T cells should represent a therapeutic target early in the course of the disease.
Methotrexate treatment suppressed cellular autoimmunity with no effect on cellular immunity against influenza in patients with RA.
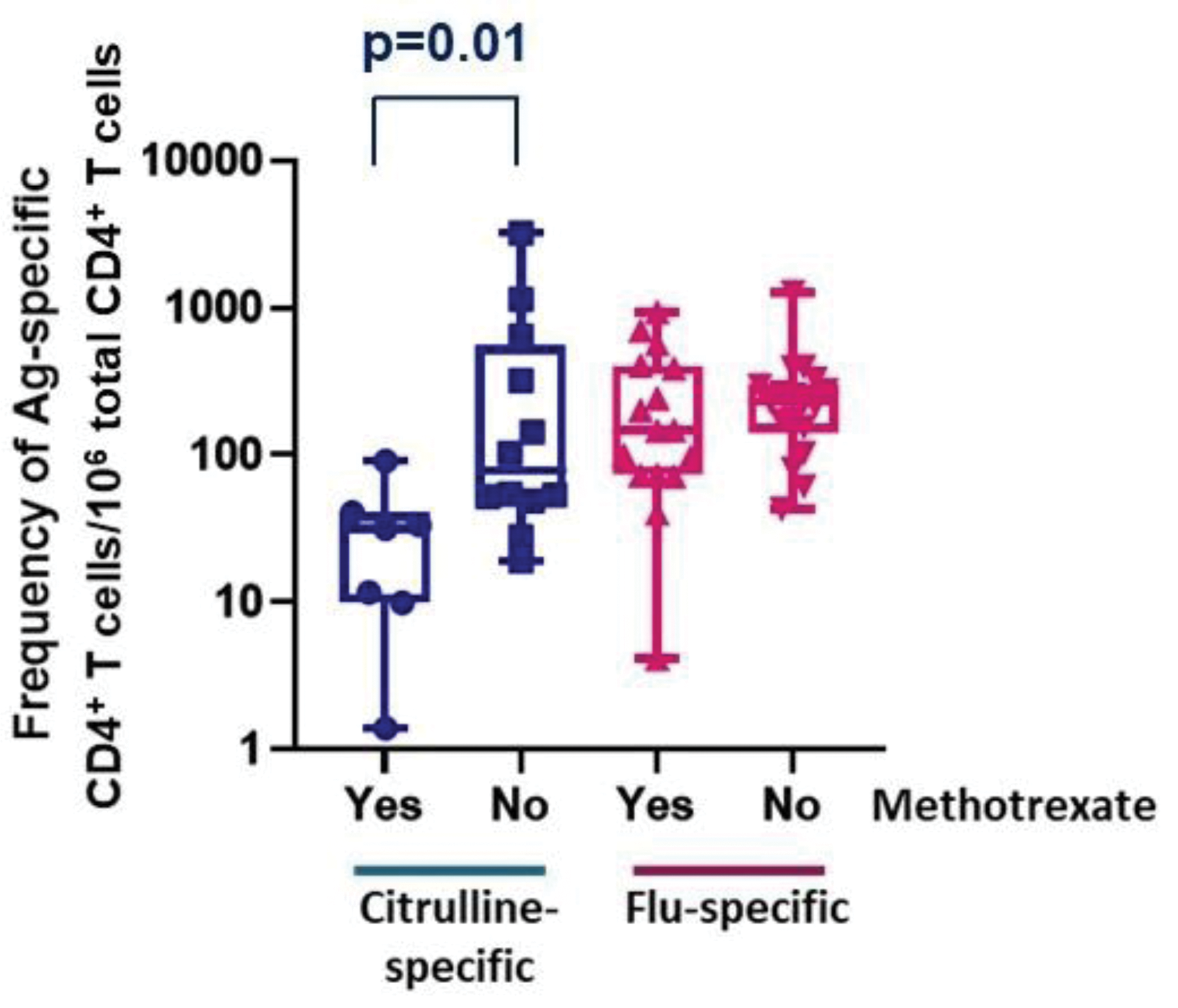
Frequency of citrulline-specific and influenza-specific CD4 + T cells in healthy HLA-DRB1*04:01 + individuals and in patients with RA before the initiation of a biologic drug.
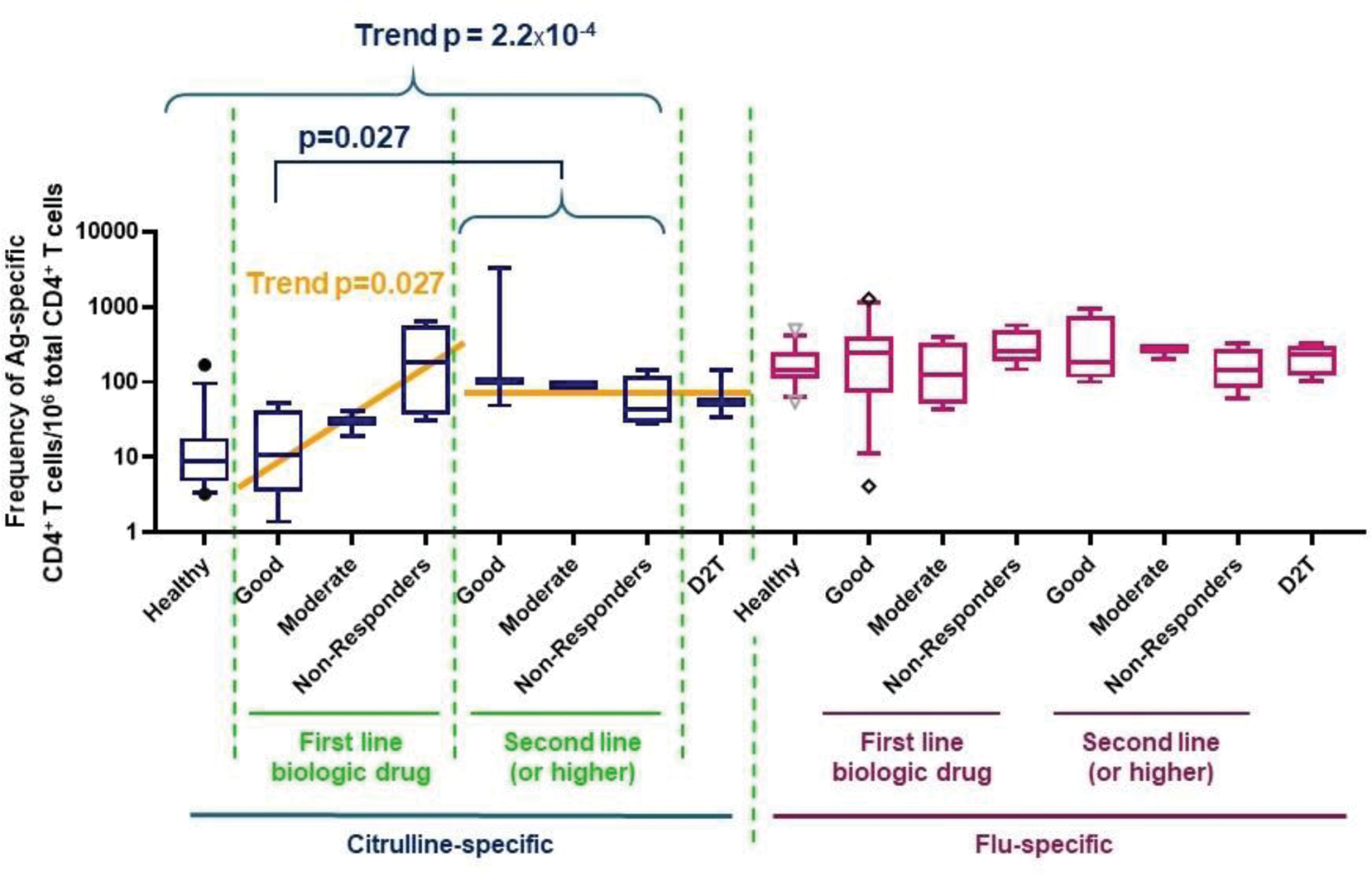
The frequency of citrulline-specific CD4 + T cells is comparable in healthy individuals and in patients receiving their first line biologic drug, provided they go on to develop a good EULAR response. D2T: difficult-to-treat RA as defined by EULAR recommendations.
REFERENCES: NIL.
Acknowledgements: Newton-Mosharafa Fund (Egyptian Ministry of Higher Education and British Council); NIHR Senior Investigator award to Anne Barton; Versus Arthritis (grant ref 21754 and 21818); NIHR Manchester BRC.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (